Anticancer Activity of New 1,2,3-Triazole-Amino Acid Conjugates
Abstract
:1. Introduction
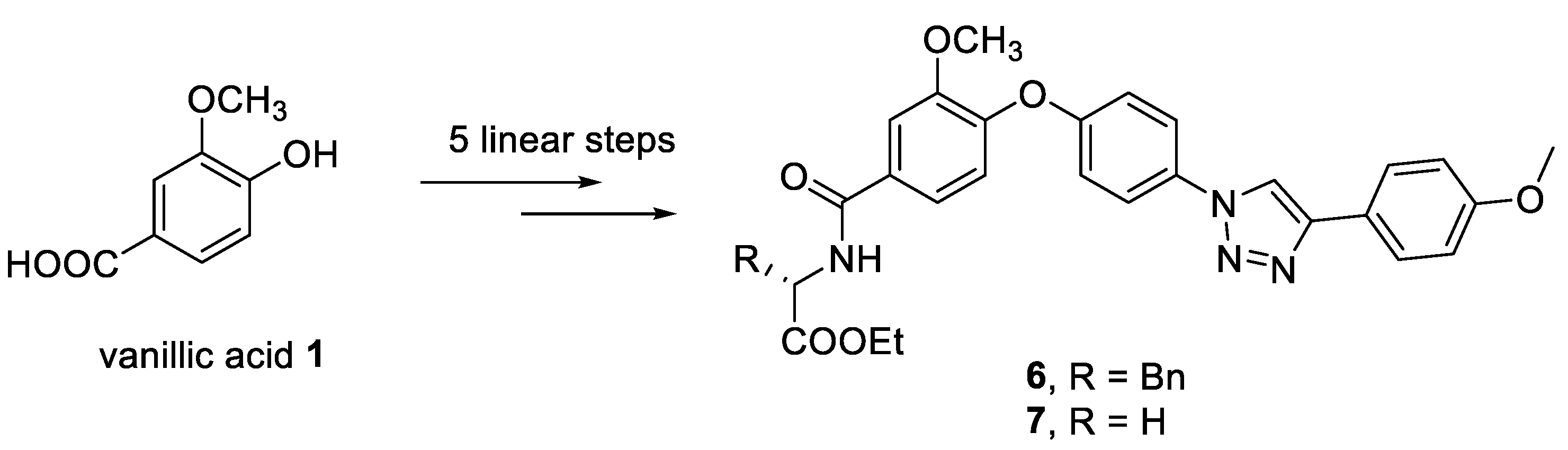
2. Results and Discussion
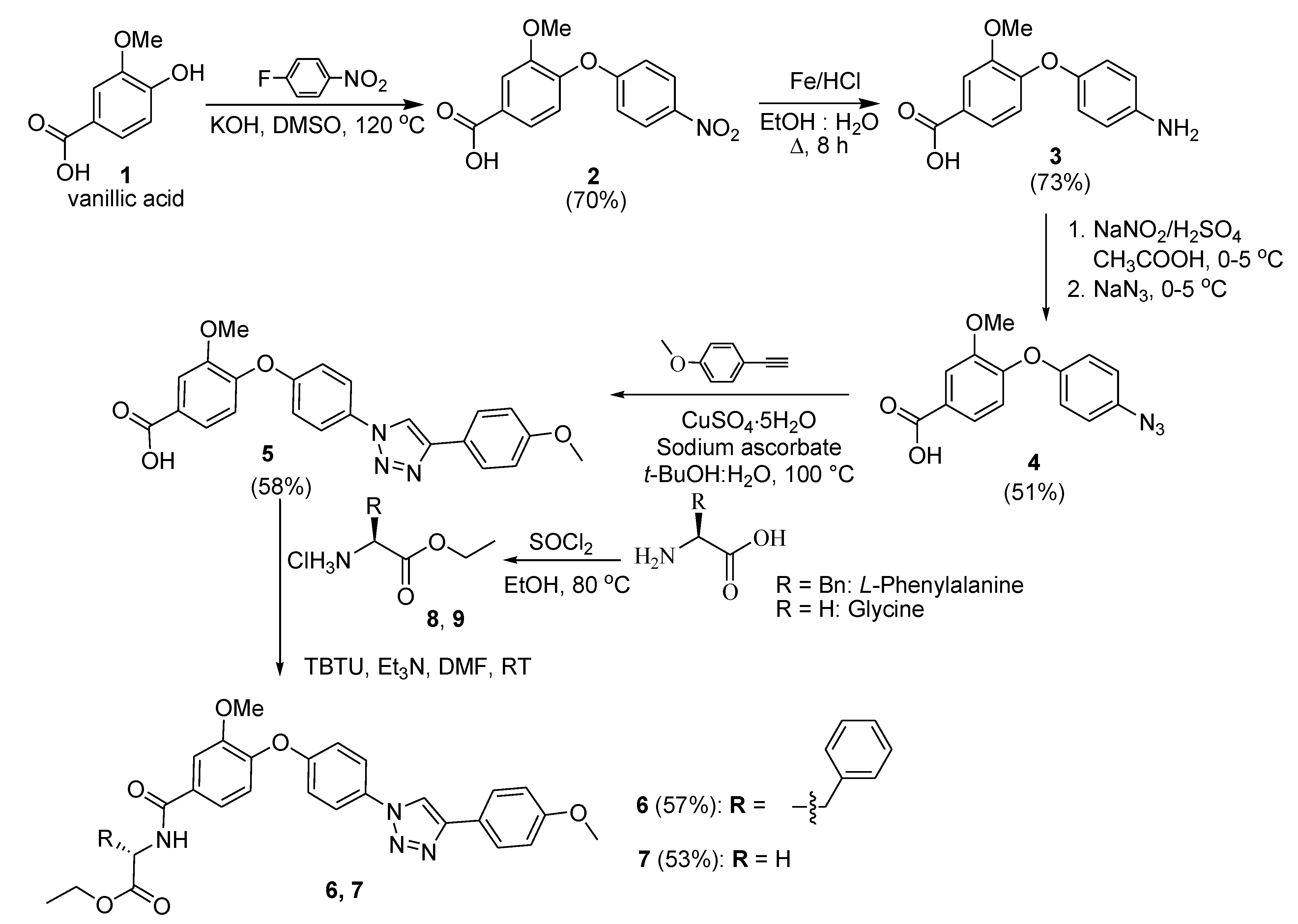
2.1. Synthesis
2.2. Anticancer Activity of 6 and 7
3. Materials and Methods
3.1. Materials
3.2. Synthesis Procedure
3.3. MTT Assay for Cell Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passsarella, D. The 1,2,3-triazole ring as bioisotere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Francesca, P.; Tracey, P.; Erika, D.G.; Riccardo, D.B.; Gian, C.T.; Giovanni, S.; Armando, A.G. Rapid synthesis of triazole-modified resveratrol analogues via click chemistry. J. Med. Chem. 2006, 49, 467–470. [Google Scholar]
- Vo, D.D.; Rouaud, I.; Devehat, F.L.; Gautier, F.; Barillé-Nion, S.; Juin, P.; Levoin, N.; Boustie, J.; Grée, R. Preliminary studies on the activity of mixed polyphenol-heterocyclic systems against B16-F10 melanoma cancer cells. Med. Chem. 2016, 12, 419–425. [Google Scholar]
- Vo, D.D.; Gautier, F.; Barillé-Nion, S.; Juin, P.; Levoin, N.; Grée, R. Design, synthesis and biological evaluation of new inhibitors of Bax/Bcl-xL in cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 1758–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lu, J.; Liu, L.; Bernard, D.; Yang, C.-Y.; Fernandez-Salas, E.; Chinnaswamy, K.; Layton, S.; Stuckey, J.; Yu, Q.; et al. A potent small-molecule inhibitor of the DCN1-UBC12 interaction that selectively blocks cullin 3 neddylation. Nat. Commun. 2017, 8, 1150. [Google Scholar] [CrossRef] [PubMed]
- Read, C.; Fitzpatrick, C.M.; Yang, P.; Kuc, R.E.; Maguire, J.J.; Glen, R.C.; Foster, R.E.; Davenport, A.P. Cardiac action of the first G protein biased small molecule apelin agonist. Biochem. Pharmacol. 2016, 116, 63–72. [Google Scholar]
- Liu, J.; Agopiantz, M.; Poupon, J.; Wu, Z.; Just, P.-A.; Borghese, B.; Segal-Bendirdjian, E.; Gauchotte, G.; Gompel, A.; Forgez, P. Neurotensin receptor 1 antagonist SR48692 improves response to carboplatin by enhancing apoptosis and inhibitiing drug efflux in ovarian cancer. Clin. Cancer Res. 2017, 23, 6516–6528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thai, D.P.T.; Le, H.N.; Le, T.T.; Vo, D.D.; Le, T.T. Synthesis of new 1,2,3-triazole derivatives from vanillin and β-naphthol. Vietnam. J. Chem. 2019, 57, 116–120. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tim, M. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Kenneth, D.P.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluable tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Dang, T.T.A.; Le, N.T.G.; Nguyen, T.Q.G.; Hoang, T.P.; Nguyen, T.A.; Mai, H.H.; Nguyen, H.T.; Le, A.T.; Nguyen, V.T. Synthesis of novel 3-arylated 2-hydroxy-1,4-naphthoquinone derivatives via microwave-assisted three-component domino reaction and evaluation of their cytotoxic activity. Chem. Heterocycl. Compd. 2021, 57, 137–142. [Google Scholar]
| Entry | KOH Equivalents | Isolated Yield (%) |
|---|---|---|
| 1 | 2 | 59 |
| 2 | 3 | 70 |
| 3 | 5 | 70 |
| Compound. | MCF7 | HepG2 | ||
|---|---|---|---|---|
| IC50 (µM) | % Inhibition at <10 µM | IC50 (µM) | % Inhibition at <10 µM | |
| 6 | 129.6 ± 3.7 | 30–40 (1.7–6.7) | 115.5 ± 2.5 | 23–32 (1.7–6.7) |
| 7 | 199.6 ± 6.0 | 28–35 (2–8) | 30.3 ± 1.6 | 29–35 (2–8) |
| Ellipticine | 2.5 ± 0.2 | - | 1.3 ± 0.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.T.; Le, P.T.K.; Dam, H.T.T.; Vo, D.D.; Le, T.T. Anticancer Activity of New 1,2,3-Triazole-Amino Acid Conjugates. Molbank 2021, 2021, M1204. https://doi.org/10.3390/M1204
Le TT, Le PTK, Dam HTT, Vo DD, Le TT. Anticancer Activity of New 1,2,3-Triazole-Amino Acid Conjugates. Molbank. 2021; 2021(2):M1204. https://doi.org/10.3390/M1204
Chicago/Turabian StyleLe, Thanh Thanh, Phung Thi Kim Le, Hai Thi Thanh Dam, Duy Duc Vo, and Thanh Tin Le. 2021. "Anticancer Activity of New 1,2,3-Triazole-Amino Acid Conjugates" Molbank 2021, no. 2: M1204. https://doi.org/10.3390/M1204
APA StyleLe, T. T., Le, P. T. K., Dam, H. T. T., Vo, D. D., & Le, T. T. (2021). Anticancer Activity of New 1,2,3-Triazole-Amino Acid Conjugates. Molbank, 2021(2), M1204. https://doi.org/10.3390/M1204






