N-[(1R)-1-(4-Chlorophenyl)ethyl]-Cyanamide
Abstract
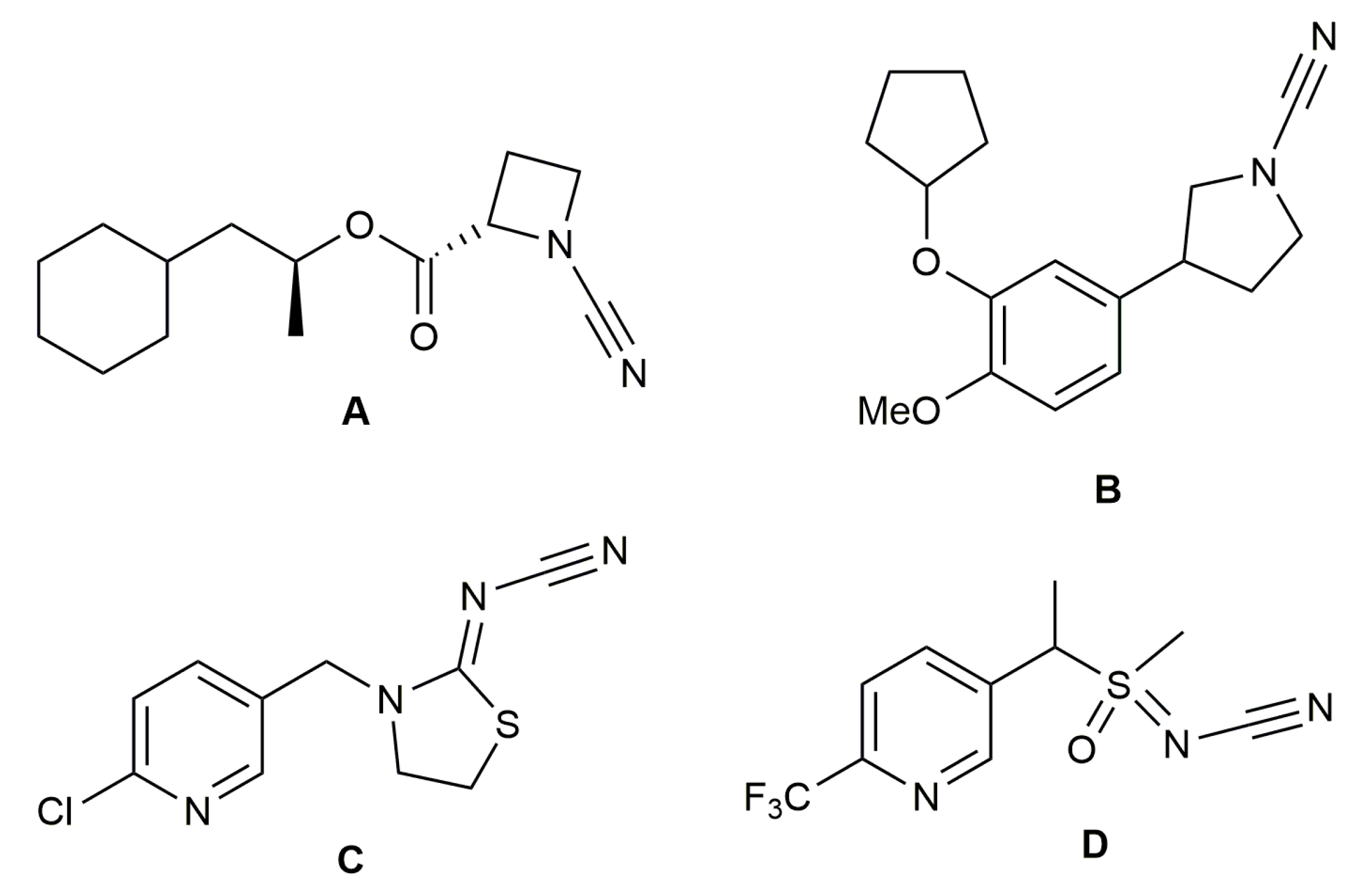
1. Introduction
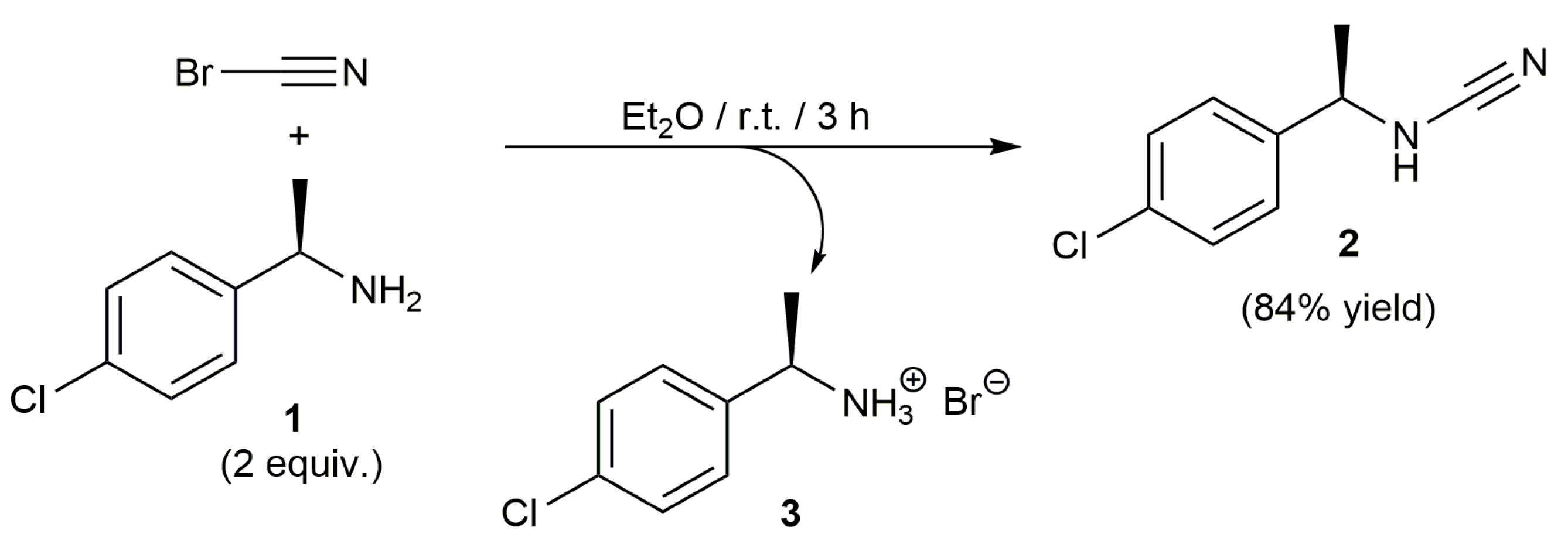
2. Results and Discussion
3. Materials and Methods
N-[(1R)-1-(4-Chlorophenyl)ethyl]-Cyanamide (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nekrasov, D.D. Synthesis and chemical transformations of mono- and disubstituted cyanamides. Russ. J. Org. Chem. 2004, 40, 1387–1402. [Google Scholar] [CrossRef]
- Larraufie, M.-H.; Maestri, G.; Malacria, M.; Ollivier, C.; Fensterbank, L.; Lacôte, E. The cyanamide moiety, synthesis and reactivity. Synthesis 2012, 44, 1279–1292. [Google Scholar]
- Prabhath, M.R.R.; Williams, L.; Bhat, S.V.; Sharma, P. Recent advances in cyanamide chemistry: Synthesis and applications. Molecules 2017, 22, 615. [Google Scholar] [CrossRef] [PubMed]
- Deaton, D.N.; Hassell, A.M.; McFadyen, R.B.; Miller, A.B.; Miller, L.R.; Shewchuk, L.S.; Tavares, F.X.; Willard, D.H.; Wright, L.L. Novel and potent cyclic cyanamide-based cathepsin K inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Feldman, P.L.; Brackeen, M.F.; Cowan, D.J.; Marron, B.E.; Schoenen, F.J.; Stafford, J.A.; Suh, E.M.; Domanico, P.L.; Rose, D.; Leesnitzer, M.A.; et al. Phosphodiesterase type IV inhibition. Structure-activity relationships of 1,3-disubstituted pyrrolidines. J. Med. Chem. 1995, 38, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Schuld, M.; Schmuck, R. Effects of thiacloprid, a new chloronicotinyl insecticide, on the egg parasitoid Trichogramma cocoeciae. Ecotoxicology 2000, 9, 197–205. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-T.; Teng, F.; Cheng, J. The construction of X-CN (X = N, S, O) bonds. Adv. Synth. Catal. 2017, 359, 26–38. [Google Scholar] [CrossRef]
- Kumar, V.; Kaushik, M.P.; Mazumdar, A. An efficient approach for the synthesis of N-1 substituted hydantoins. Eur. J. Org. Chem. 2008, 2008, 1910–1916. [Google Scholar] [CrossRef]
- Cockerill, A.F.; Deacon, A.; Harrison, R.G.; Osborne, D.J.; Prime, D.M.; Ross, W.J.; Todd, A.; Verge, J.P. An improved synthesis of 2-amino-1,3-oxazoles under basic catalysis. Synthesis 1976, 1976, 591–593. [Google Scholar] [CrossRef]
- Guijarro, D.; Pablo, Ó.; Yus, M. Asymmetric synthesis of chiral primary amines by transfer hydrogenation of N-(tert-butanesulfinyl)ketimines. J. Org. Chem. 2010, 75, 5265–5270. [Google Scholar] [CrossRef]
- Niwa, R.; Kamada, H.; Shitara, E.; Horiuchi, J.; Kibushi, N.; Kato, T. Synthesis of isomelamines and isocyanurates and their biological evaluation. Chem. Pharm. Bull. 1996, 44, 2314–2317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Luttrell, W.E. Cyanogen bromide. J. Chem. Health Saf. 2009, 16, 29–30. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Fernández, R.; Crochet, P.; Cadierno, V. N-[(1R)-1-(4-Chlorophenyl)ethyl]-Cyanamide. Molbank 2021, 2021, M1198. https://doi.org/10.3390/M1198
González-Fernández R, Crochet P, Cadierno V. N-[(1R)-1-(4-Chlorophenyl)ethyl]-Cyanamide. Molbank. 2021; 2021(2):M1198. https://doi.org/10.3390/M1198
Chicago/Turabian StyleGonzález-Fernández, Rebeca, Pascale Crochet, and Victorio Cadierno. 2021. "N-[(1R)-1-(4-Chlorophenyl)ethyl]-Cyanamide" Molbank 2021, no. 2: M1198. https://doi.org/10.3390/M1198
APA StyleGonzález-Fernández, R., Crochet, P., & Cadierno, V. (2021). N-[(1R)-1-(4-Chlorophenyl)ethyl]-Cyanamide. Molbank, 2021(2), M1198. https://doi.org/10.3390/M1198






