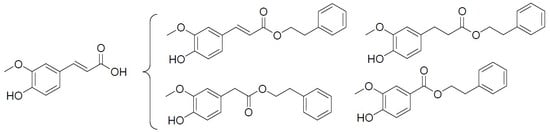
Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors
Abstract
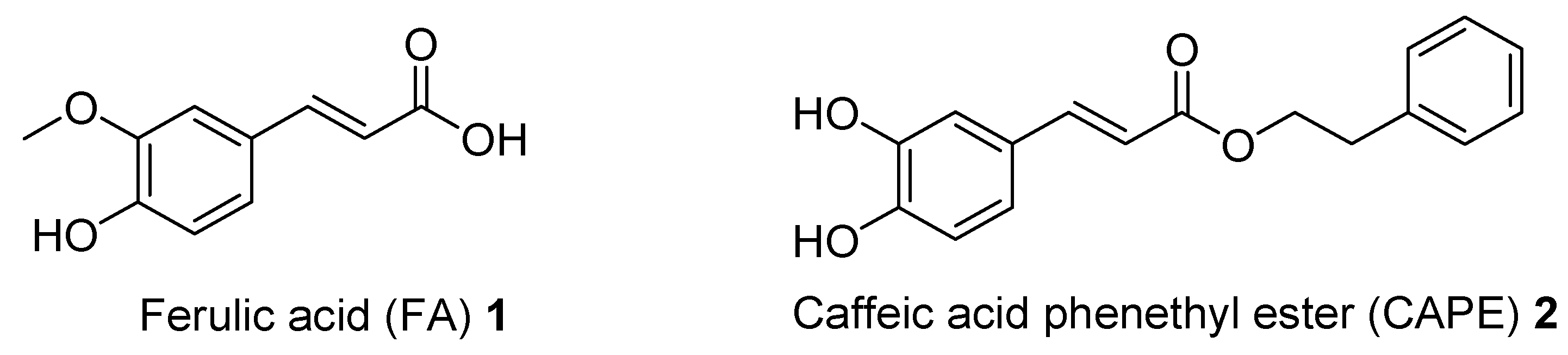
1. Introduction
2. Results
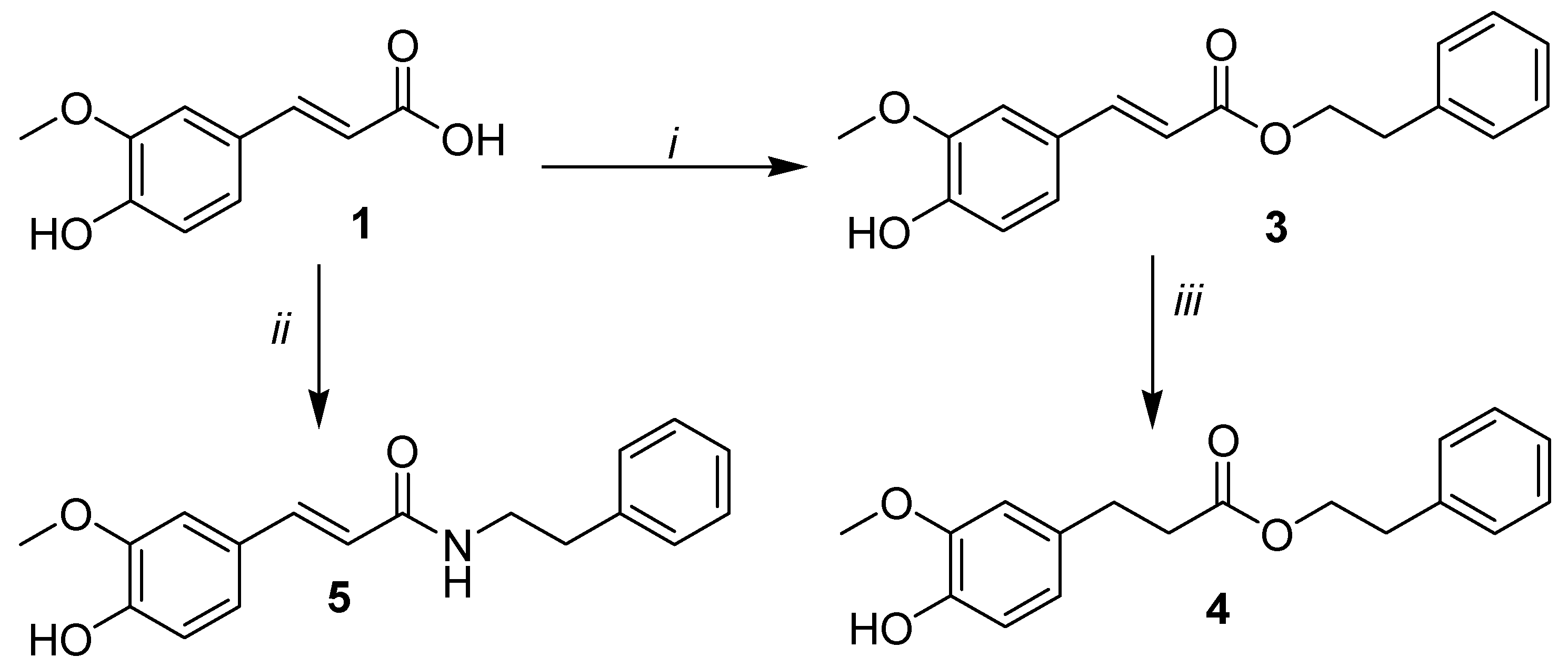
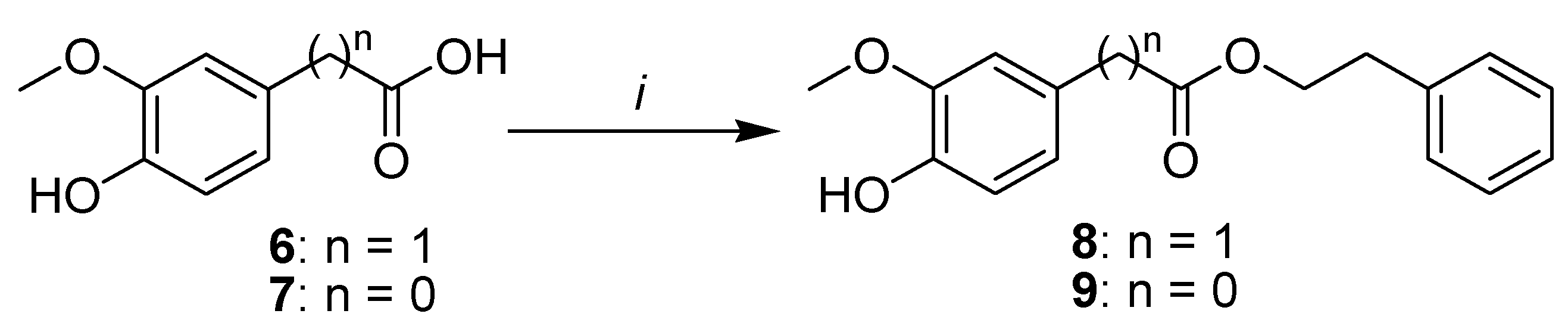
2.1. Synthesis
2.2. Free Radical Scavenging Activity Assay
2.3. In Silico Physicochemical Properties and Drug Likeness Evaluation
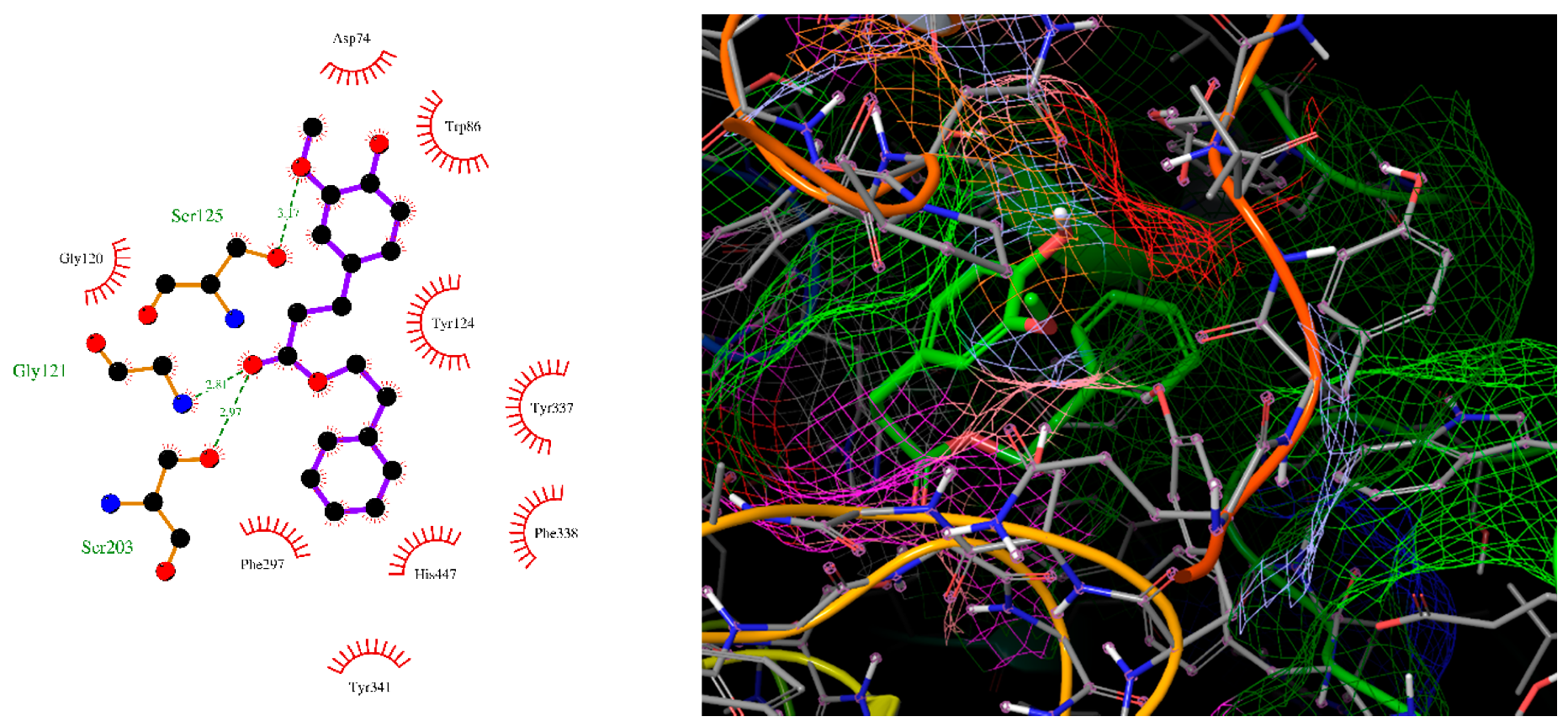
2.4. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Esterification of Phenolic Acids.
2-Phenylethyl (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate (3)
Phenethyl 3-(4-hydroxy-3-methoxyphenyl)propanoate (4)
3-(4-Hydroxy-3-methoxyphenyl)-N-phenethylacrylamide (5)
Phenethyl 2-(4-hydroxy-3-methoxyphenyl)acetate (8)
Phenethyl 4-hydroxy-3-methoxybenzoate (9)
3.2. Free Radical Scavenging Activity Assay
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dassel, K.; Butler, J.; Telonidis, J.; Edelman, L. Development and evaluation of Alzheimer’s Disease and Related Dementias (ADRD) best care practices in long-term care online training program. Educ. Gerontol. 2020, 46, 150–157. [Google Scholar] [CrossRef]
- Wan, T.; Wang, Z.; Luo, Y.; Zhang, Y.; He, W.; Mei, Y.; Xue, J.; Li, M.; Pan, H.; Li, W.; et al. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Protects against Oxidative Stress-Mediated Neuronal Cell Apoptosis and Scopolamine-Induced Cognitive Impairment by Activating Nrf2/HO-1 Signaling. Oxidative Med. Cell. Longev. 2019, 2019, 8239642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in A Mouse Model of Alzheimer’s Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018, 9, 605–622. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative Stress and β-Amyloid Protein in Alzheimer’s Disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Dinamarca, M.C.; Alvarez, A. Amyloid–cholinesterase interactions. FEBS J. 2008, 275, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, M.; Chen, Z.; Wang, S.; Lapucci, A.; Macchia, M.; Yang, X.; Chu, J.; Han, Y.; Pi, R.; Rapposelli, S. Synthesis and pharmacological evaluation of multifunctional tacrine derivatives against several disease pathways of AD. Bioorg. Med. Chem. Lett. 2015, 25, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Bovina, E.V.; Ustyugov, A.A. Drugs in Clinical Trials for Alzheimer’s Disease: The Major Trends. Med. Res. Rev. 2017, 37, 1186–1225. [Google Scholar] [CrossRef] [PubMed]
- Benchekroun, M.; Pachón-Angona, I.; Luzet, V.; Martin, H.; Oset-Gasque, M.-J.; Marco-Contelles, J.; Ismaili, L. Synthesis, antioxidant and Aβ anti-aggregation properties of new ferulic, caffeic and lipoic acid derivatives obtained by the Ugi four-component reaction. Bioorg. Chem. 2019, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- He, X.-x.; Yang, X.-h.; Ou, R.-y.; Ouyang, Y.; Wang, S.-n.; Chen, Z.-w.; Wen, S.-j.; Pi, R.-b. Synthesis and evaluation of multifunctional ferulic and caffeic acid dimers for Alzheimer’s disease. Nat. Prod. Res. 2017, 31, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Cho, J.-y.; Kim, D.-H.; Yan, J.-J.; Lee, H.-K.; Suh, H.-W.; Song, D.-K. Inhibitory Effects of Long-Term Administration of Ferulic Acid on Microglial Activation Induced by Intracerebroventricular Injection of β-Amyloid Peptide (1—42) in Mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kaur, D.; Bansal, N. Caffeic Acid Phenethyl Ester (CAPE) Prevents Development of STZ-ICV Induced dementia in Rats. Pharm. Mag 2017, 13, S10–S15. [Google Scholar]
- DeBay, D.R.; Reid, G.A.; Macdonald, I.R.; Mawko, G.; Burrell, S.; Martin, E.; Bowen, C.V.; Darvesh, S. Butyrylcholinesterase-knockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer’s disease. Brain Res. 2017, 1671, 102–110. [Google Scholar] [CrossRef]
- Appendino, G.; Minassi, A.; Daddario, N.; Bianchi, F.; Tron, G.C. Chemoselective Esterification of Phenolic Acids and Alcohols. Org. Lett. 2002, 4, 3839–3841. [Google Scholar] [CrossRef]
- Shi, Z.-H.; Li, N.-G.; Shi, Q.-P.; Hao, T.; Tang, Y.-P.; Wei, L.; Lian, Y.; Yang, J.-P.; Duan, J.-A. Design, Synthesis and Biological Evaluation of Ferulic Acid Amides as Selective Matrix Metalloproteinase Inhibitors. Med. Chem. 2013, 9, 947–954. [Google Scholar]
- Padurariu, M.; Ciobica, A.; Lefter, R.; Lacramioara Serban, I.; Stefanescu, C.; Chirita, R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr. Danub. 2013, 25, 401–409. [Google Scholar]
- Parveen, M.; Aslam, A.; Nami, S.A.A.; Malla, A.M.; Alam, M.; Lee, D.-U.; Rehman, S.; Silva, P.S.P.; Silva, M.R. Potent acetylcholinesterase inhibitors: Synthesis, biological assay and docking study of nitro acridone derivatives. J. Photochem. Photobiol. B Biol. 2016, 161, 304–311. [Google Scholar] [CrossRef]
- Dighe, S.N.; Deora, G.S.; De la Mora, E.; Nachon, F.; Chan, S.; Parat, M.-O.; Brazzolotto, X.; Ross, B.P. Discovery and Structure–Activity Relationships of a Highly Selective Butyrylcholinesterase Inhibitor by Structure-Based Virtual Screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar]
- Lee, S.; Barron, M.G. Development of 3D-QSAR Model for Acetylcholinesterase Inhibitors Using a Combination of Fingerprint, Molecular Docking, and Structure-Based Pharmacophore Approaches. Toxicol. Sci. 2015, 148, 60–70. [Google Scholar] [CrossRef]
- Ambure, P.; Kar, S.; Roy, K. Pharmacophore mapping-based virtual screening followed by molecular docking studies in search of potential acetylcholinesterase inhibitors as anti-Alzheimer’s agents. Biosystems 2014, 116, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hamulakova, S.; Janovec, L.; Hrabinova, M.; Spilovska, K.; Korabecny, J.; Kristian, P.; Kuca, K.; Imrich, J. Synthesis and Biological Evaluation of Novel Tacrine Derivatives and Tacrine–Coumarin Hybrids as Cholinesterase Inhibitors. J. Med. Chem. 2014, 57, 7073–7084. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Gallelli, A.; Manini, M.; Anzini, M.; Mennuni, L.; Makovec, F.; Menziani, M.C.; Alcaro, S.; Ortuso, F.; Vomero, S. Further Studies on the Interaction of the 5-Hydroxytryptamine3 (5-HT3) Receptor with Arylpiperazine Ligands. Development of a New 5-HT3 Receptor Ligand Showing Potent Acetylcholinesterase Inhibitory Properties. J. Med. Chem. 2005, 48, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.-P.; Gobec, S. Discovery, Biological Evaluation, and Crystal Structure of a Novel Nanomolar Selective Butyrylcholinesterase Inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef]
- Cheung, J.; Gary, E.N.; Shiomi, K.; Rosenberry, T.L. Structures of Human Acetylcholinesterase Bound to Dihydrotanshinone I and Territrem B Show Peripheral Site Flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef]
- Rosenberry, T.L.; Brazzolotto, X.; Macdonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the binding of reversible inhibitors to human butyrylcholinesterase and acetylcholinesterase: A crystallographic, kinetic and calorimetric study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept®): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Kryger, G.; Harel, M.; Giles, K.; Toker, L.; Velan, B.; Lazar, A.; Kronman, C.; Barak, D.; Ariel, N.; Shafferman, A.; et al. Structures of recombinant native and E202Q mutant human acetylcholinesterase complexed with the snake-venom toxin fasciculin-II. Acta Crystallogr. Sect. D 2000, 56, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-Y.; Weng, T.-T.; Lin, G.-Z.; Lu, R.-J.; Jian, S.-Y.; Lin, G. Molecular docking of different inhibitors and activators to butyrylcholinesterase. J. Biomol. Struct. Dyn. 2015, 33, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.C.; Rudolph, M.J.; Ginter, C.; Cassidy, M.S.; Cheung, J. Structures of paraoxon-inhibited human acetylcholinesterase reveal perturbations of the acyl loop and the dimer interface. Proteins Struct. Funct. Bioinform. 2016, 84, 1246–1256. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Modeling 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Schrödinger. Schrödinger Maestro, Release, 2016-3; Schrödinger Inc.: New York, NY, USA, 2016. [Google Scholar]
| Compounds | IC50 (µM) [SEM] |
|---|---|
| FA (1) | 23.93 ± 0.09 |
| 3 | 49.99 ± 0.04 |
| 4 | 39.41± 0.06 |
| 5 | 40.00 ± 0.07 |
| 8 | 53.63 ± 0.06 |
| 9 | 27.95 ± 0.18 |
| ascorbic acid | 12.68 ± 0.02 |
| Physicochemical Properties | Lipophilicity | Pharmacokinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| MW (g/mol) | ROTB (n) | HBA (n) | HBD (n) | TPSA (Å) | CLogPo/w | GIA | BBBP | |
| Rule | <500 | ≤10 | <10 | <5 | ≤140 | <5 | - | - |
| FA (1) | 194.18 | 3 | 4 | 2 | 66.76 | 1.36 | High | Yes |
| 3 | 298.33 | 7 | 4 | 1 | 55.76 | 3.40 | High | Yes |
| 4 | 300.35 | 8 | 4 | 1 | 55.76 | 3.13 | High | Yes |
| 5 | 297.35 | 7 | 3 | 2 | 58.56 | 2.90 | High | Yes |
| 8 | 286.32 | 7 | 4 | 1 | 55.76 | 2.95 | High | Yes |
| 9 | 272.30 | 6 | 4 | 1 | 55.76 | 3.09 | High | Yes |
| Ligand | Affinity (kcal/mol) | H-Bonds | π–π Interactions | |
|---|---|---|---|---|
| AChE (4EY4) | FA (1) | −7.5 | Asp74, Tyr337, Tyr341 | Trp86 |
| 3 | −9.2 | Trp286 | Trp86, Tyr337 | |
| 4 | −10.2 | Gly121, Ser125, Ser203 | Trp86, Phe338, Tyr341 | |
| 5 | −8.8 | Asp74, Trp286 × 2 | Trp86, Tyr337 | |
| 8 | −9.7 | Tyr124, Ser125 | Trp86, Tyr337, Phe338 | |
| 9 | −9.8 | Arg296 × 2 | Trp286, Tyr337, Tyr341 | |
| BChE (1P0I) | FA (1) | −6.8 | Asp70, Tyr128, Gly197, Tyr332 × 2 | Trp82 |
| 3 | −9.0 | Tyr128, Gly197 | Trp82, Tyr332 | |
| 4 | −9.0 | Trp82, Tyr128, Tyr440 | Trp82, Tyr332 | |
| 5 | −8.6 | Pro285, Ser198 × 2, His438 | Trp231, Phe329, Tyr332 | |
| 8 | −8.7 | His438 | Trp231, Trp329 | |
| 9 | −8.4 | Glu197 | Trp82, Tyr332 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selka, A.; Ndongou Moutombi, F.J.; Cormier, M.; Touaibia, M. Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors. Molbank 2020, 2020, M1151. https://doi.org/10.3390/M1151
Selka A, Ndongou Moutombi FJ, Cormier M, Touaibia M. Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors. Molbank. 2020; 2020(3):M1151. https://doi.org/10.3390/M1151
Chicago/Turabian StyleSelka, Ayyoub, Fanta J. Ndongou Moutombi, Marc Cormier, and Mohamed Touaibia. 2020. "Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors" Molbank 2020, no. 3: M1151. https://doi.org/10.3390/M1151
APA StyleSelka, A., Ndongou Moutombi, F. J., Cormier, M., & Touaibia, M. (2020). Phenethyl Esters and Amide of Ferulic Acid, Hydroferulic Acid, Homovanillic Acid, and Vanillic Acid: Synthesis, Free Radicals Scavenging Activity, and Molecular Modeling as Potential Cholinesterases Inhibitors. Molbank, 2020(3), M1151. https://doi.org/10.3390/M1151