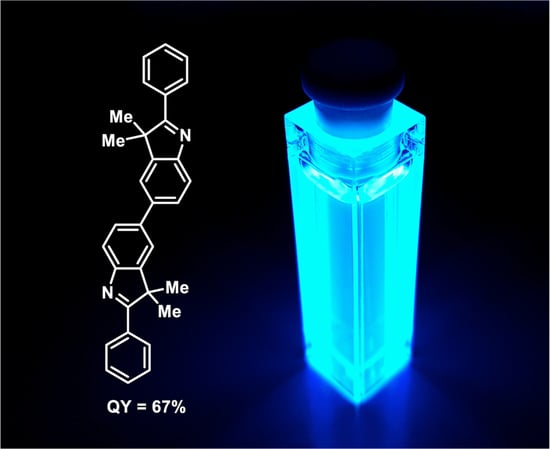
3,3,3′,3′-Tetramethyl-2,2′-diphenyl-3H,3′H-5,5′-biindole
Abstract
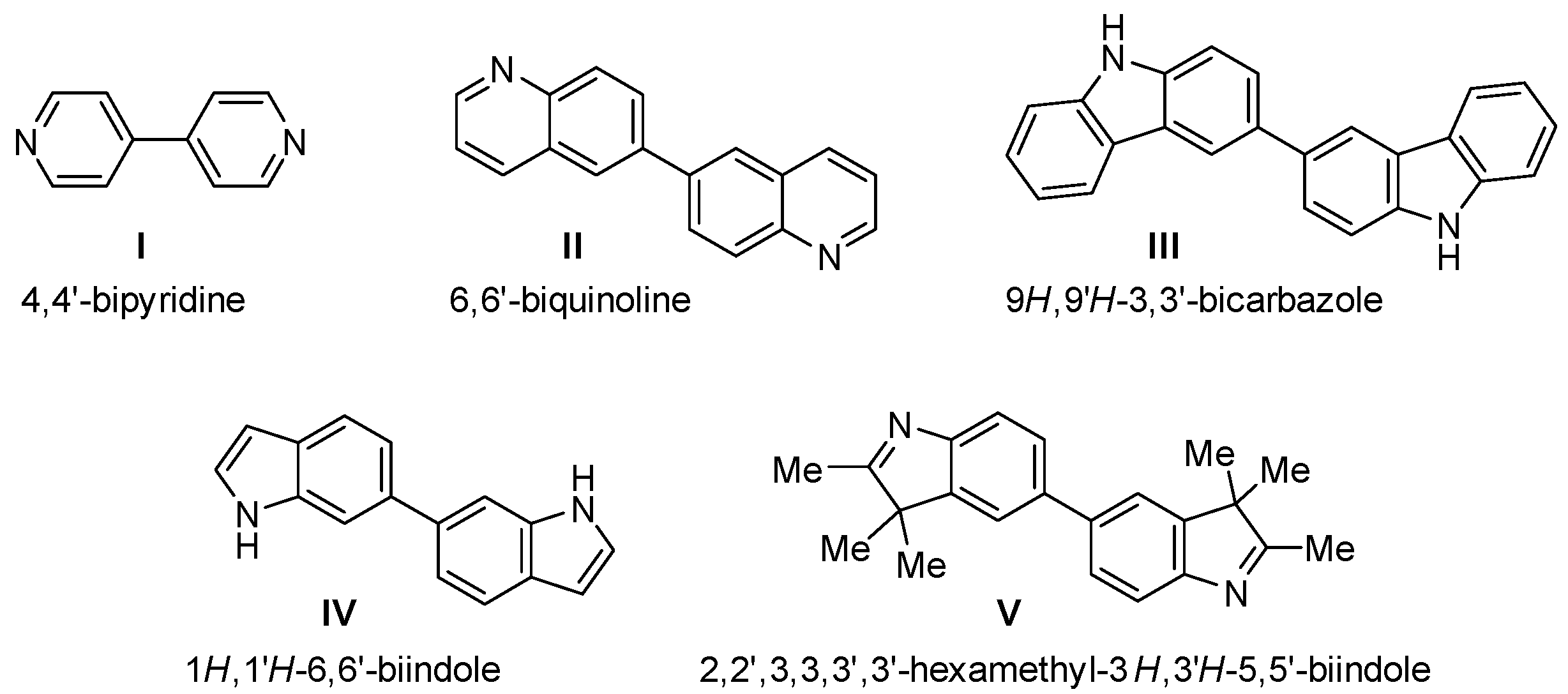
1. Introduction
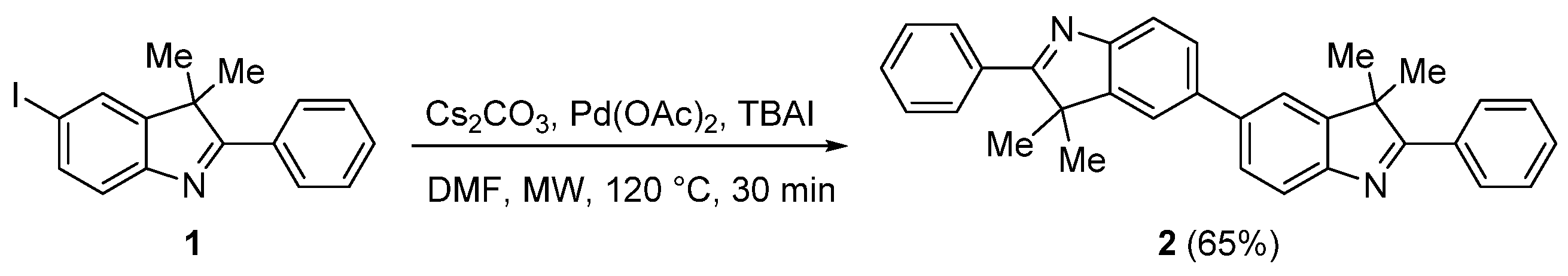
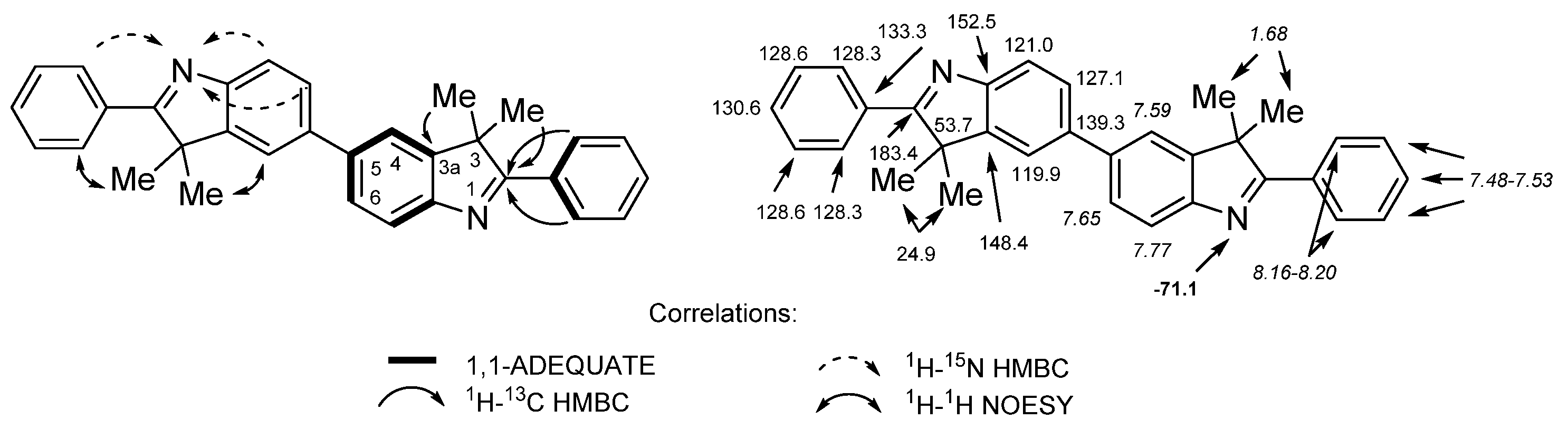
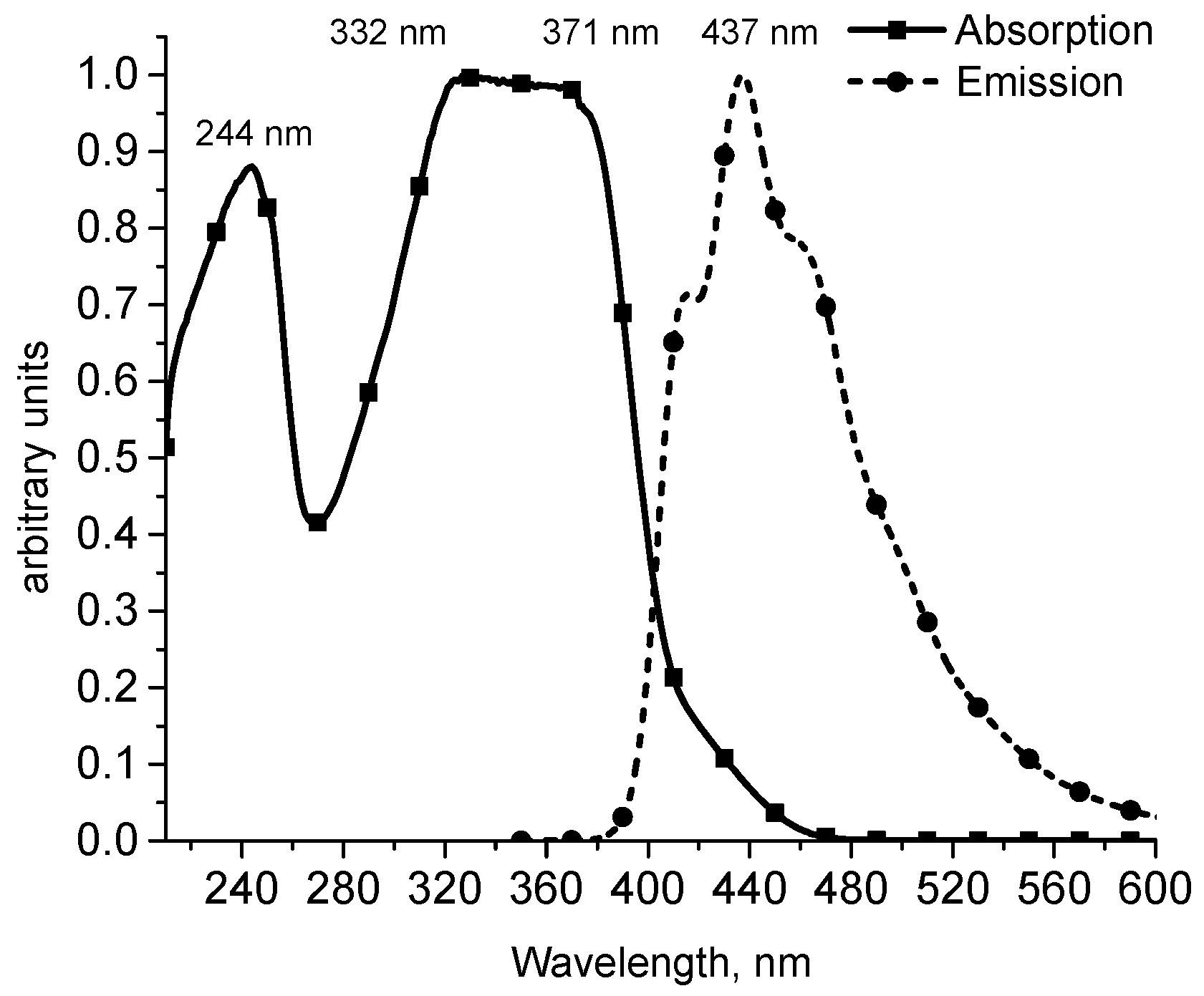
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kozlowski, M.C.; Morgan, B.J.; Linton, E.C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 2009, 38, 3193–3207. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Asmar, R. Telmisartan in high cardiovascular risk patients. Eur. Cardiol. 2012, 8, 10–16. [Google Scholar] [CrossRef]
- Van der Bom, T.; Winter, M.M.; Bouma, B.J.; Groenink, M.; Vliegen, H.W.; Pieper, P.G.; van Dijk, A.P.J.; Sieswerda, G.T.; Roos-Hesselink, J.W.; Zwinderman, A.H.; et al. Effect of valsartan on systemic right ventricular function: A double-blind, randomized, placebo-controlled pilot trial. Circulation 2013, 127, 322–330. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Activity of boscalid, fenhexamid, fluazinam, fludioxonil, and vinclozolin on growth of Sclerotinia minor and S. sclerotiorum and development of lettuce drop. Plant Dis. 2004, 88, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Huang, W.S.; Pu, L. Biaryl-based macrocyclic and polymeric chiral (salophen)Ni(II) complexes: Synthesis and spectroscopic study. J. Org. Chem. 2001, 66, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Bryleva, Y.A.; Glinskaya, L.A.; Antonova, O.V.; Kokina, T.E.; Larionov, S.V. Coordination polymer [Sm(Biq)(iso-Bu2PS2)3]n: Synthesis, structure, and photoluminescence. Russ. J. Coord. Chem. Khimiya 2014, 40, 184–188. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, C.H.; Godumala, M.; Kim, C.Y.; Kim, H.J.; Hwang, J.H.; Kim, Y.W.; Choi, D.H.; Cho, M.J.; Choi, D.H. Rational design, synthesis, and characterization of a photocrosslinkable hole-transporting polymer for high performance solution-processed thermally activated delayed fluorescence OLEDs. J. Mater. Chem. C 2020, 8, 4572–4579. [Google Scholar] [CrossRef]
- Zhou, G.; Sofiyev, V.; Kaur, H.; Snyder, B.A.; Mankowski, M.K.; Hogan, P.A.; Ptak, R.G.; Gochin, M. Structure-activity relationship studies of indole-based compounds as small molecule HIV-1 fusion inhibitors targeting glycoprotein 41. J. Med. Chem. 2014, 57, 5270–5281. [Google Scholar] [CrossRef]
- Lee, S.; Ji, S.; Kang, Y.J. Synthesis and characterizations of bis-spiropyran derivatives. Bull. Korean Chem. Soc. 2012, 33, 3740–3744. [Google Scholar] [CrossRef][Green Version]
- Steponavičiūtė, R.; Martynaitis, V.; Bieliauskas, A.; Šačkus, A. Synthesis of new fluorescent building blocks via the microwave-assisted annulation reaction of 1,1,2-trimethyl-1H-benzo[e]indole with acrylic acid and its derivatives. Tetrahedron 2014, 70, 1967–1974. [Google Scholar] [CrossRef]
- Buinauskaitė, V.; Martynaitis, V.; Mangelinckx, S.; Kreiza, G.; De Kimpe, N.; Šačkus, A. Facile synthesis of spiro[benzo[e]indole-2,2′-piperidine] derivatives and their transformation to novel fluorescent scaffolds. Tetrahedron 2012, 68, 9260–9266. [Google Scholar] [CrossRef]
- Ragaitė, G.; Martynaitis, V.; Kriščiūnienė, V.; Kleizienė, N.; Redeckas, K.; Voiciuk, V.; Vengris, M.; Šačkus, A. Fast and stable light-driven molecular switch based on a 5a,13-methanoindolo [2,1-b][1,3]benzoxazepine ring system. Dye. Pigment. 2015, 113, 546–553. [Google Scholar] [CrossRef]
- Žukauskaitė, Ž.; Buinauskaitė, V.; Solovjova, J.; Malinauskaitė, L.; Kveselytė, A.; Bieliauskas, A.; Ragaitė, G.; Šačkus, A. Microwave-assisted synthesis of new fluorescent indoline-based building blocks by ligand free Suzuki-Miyaura cross-coupling reaction in aqueous media. Tetrahedron 2016, 72, 2955–2963. [Google Scholar] [CrossRef]
- Voiciuk, V.; Redeckas, K.; Martynaitis, V.; Steponavičiūtė, R.; Šačkus, A.; Vengris, M. Improving the photochromic properties of indolo[2,1-b][1,3]benzoxazines with phenylic substituents. J. Photochem. Photobiol. A Chem. 2014, 278, 60–68. [Google Scholar] [CrossRef]
- Buinauskaitė, V.; Dargytė, M.; Šačkus, A. Synthesis and microwave-promoted Suzuki-Miyaura cross-coupling reactions of 5-bromo-1,3,3-trimethyl-1,3-dihydro-6ʹH-spiro[indole-2,2ʹ-piperidin]-6ʹ-one. Chemija 2012, 23, 322–327. [Google Scholar]
- Yin, L.; Liebscher, J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Fanta, P.E. The Ullmann Synthesis of Biaryls, 1945-1963. Chem. Rev. 1964, 64, 613–632. [Google Scholar] [CrossRef]
- Stefani, H.A.; Guarezemini, A.S.; Cella, R. Homocoupling reactions of alkynes, alkenes and alkyl compounds. Tetrahedron 2010, 66, 7871–7918. [Google Scholar] [CrossRef]
- Vasconcelos, S.N.S.; Reis, J.S.; de Oliveira, I.M.; Balfour, M.N.; Stefani, H.A. Synthesis of symmetrical biaryl compounds by homocoupling reaction. Tetrahedron 2019, 75, 1865–1959. [Google Scholar] [CrossRef]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Tomasulo, M.; Salvatore Sortino, A.; Françisco, M. Raymo Bichromophoric Photochromes Based on the Opening and Closing of a Single Oxazine Ring. J. Org. Chem. 2008, 73, 118–126. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šachlevičiūtė, U.; Varvuolytė, G.; Bieliauskas, A.; Kleizienė, N.; Žukauskaitė, A.; Šačkus, A. 3,3,3′,3′-Tetramethyl-2,2′-diphenyl-3H,3′H-5,5′-biindole. Molbank 2020, 2020, M1146. https://doi.org/10.3390/M1146
Šachlevičiūtė U, Varvuolytė G, Bieliauskas A, Kleizienė N, Žukauskaitė A, Šačkus A. 3,3,3′,3′-Tetramethyl-2,2′-diphenyl-3H,3′H-5,5′-biindole. Molbank. 2020; 2020(3):M1146. https://doi.org/10.3390/M1146
Chicago/Turabian StyleŠachlevičiūtė, Urtė, Gabrielė Varvuolytė, Aurimas Bieliauskas, Neringa Kleizienė, Asta Žukauskaitė, and Algirdas Šačkus. 2020. "3,3,3′,3′-Tetramethyl-2,2′-diphenyl-3H,3′H-5,5′-biindole" Molbank 2020, no. 3: M1146. https://doi.org/10.3390/M1146
APA StyleŠachlevičiūtė, U., Varvuolytė, G., Bieliauskas, A., Kleizienė, N., Žukauskaitė, A., & Šačkus, A. (2020). 3,3,3′,3′-Tetramethyl-2,2′-diphenyl-3H,3′H-5,5′-biindole. Molbank, 2020(3), M1146. https://doi.org/10.3390/M1146