Abstract
3′,5′-O-Bis(tert-butyldimethylsilyl)-8-fluoro-N-2-isobutyryl-2′-deoxyguanosine was synthesized from 3′,5′-O-bis(tert-butyldimethylsilyl)-N-2-isobutyryl-2′-deoxyguanosine by the treatment with N-fluorobenzenesulfonimide. A similar fluorination reaction with 3′,5′-O-bis(tert-butyldimethylsilyl)-N-2-(N,N-dimethylformamidine)-2′-deoxyguanosine, however, failed to give the corresponding fluorinated product. It was found that 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine is labile under acidic conditions, but sufficiently stable in dichloroacetic acid used in solid phase synthesis. Incorporation of 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine into oligonucleotides through the phosphoramidite chemistry-based solid phase synthesis failed to give the desired products. Furthermore, treatment of 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine with aqueous ammonium hydroxide did not give 8-fluoro-2′-deoxyguanosine, but led to the formation of a mixture consisting of 8-amino-N-2-isobutyryl-2′-deoxyguanosine and C8:5′-O-cyclo-2′-deoxyguanosine. Taken together, an alternative N-protecting group and possibly modified solid phase synthetic cycle conditions will be required for the incorporation of 8-fluoro-2′-deoxyguanosine into oligonucleotides through the phosphoramidite chemistry-based solid phase synthesis.
1. Introduction
Among the numerous modifications to nucleic acids, introduction of fluorine has attracted significant interest due to its small size and the ability for stereochemical control [1]. In this respect, presence of fluorine in the sugar residues allows for the control over sugar puckers, while introduction of fluorine to the nucleobases enables unique hydrogen bonding properties. Furthermore, presence of fluorine in nucleic acids presents an opportunity to study the structures of nucleic acids and the interaction involving these molecules by 19F NMR spectroscopy due to the abundance of 19F among its isotopes, large gyromagnetic ratio, and the sensitivity of 19F chemical shifts to structural changes [2]. We recently demonstrated that 5-fluoro-2′-deoxycytidine 1 (Figure 1) is a useful probe for the study of the B/Z-DNA handedness switch by 19F NMR [3].
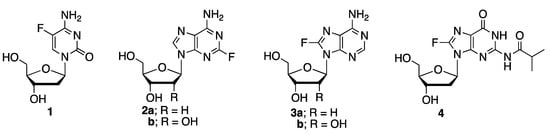
Figure 1.
Nucleosides with fluorine modification in the nucleobase.
We are interested in expanding the scope of fluorine modification of nucleobases, especially to the guanine base in this respect, as d(CG) repeats are the most widely studied sequences for the DNA handedness switch. We wish to describe the chemical synthesis of 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 and its reactivity under the conditions for the assembly of oligonucleotides through the phosphoramidite chemistry-based solid phase.
2. Results and Discussion
Syntheses of adenine nucleosides, such as 2-fluoro-2′-deoxyadenosine 2a [4,5,6,7] and 2-fluoroadenosine 2b [6,8] have been demonstrated through different approaches. Introduction of fluorine to the C-8 position of purine nucleosides is more challenging, likely due to the instability of the resulting nucleosides. The F-C bond in 8-F-rA 3b is labile under basic conditions. In this respect, basic conditions that are required for the removal of N-acyl protecting groups can lead to defluorination. Thus, an early attempt to synthesize 8-fluoroadenosine 3b by the treatment of 2′,3′,5′-O-triacetyl-8-fluoroadenosine with methanolic ammonia [9] was later found not to give the desired 8-F-rA [10]. Enzymatic [11] or deprotection conditions under mild acidic conditions [12] allowed for the access of 8-F-rA. Fluorination of guanosine at the C-8 position was demonstrated by treatment with elemental fluorine [13]. This method, however, is inconvenient due to the difficulty in handling elemental fluorine. More recently, syntheses of protected 8-fluoro deoxyinosine, deoxyadenosine (which led to the formation of 8-fluoro-2′-deoxyadenosine 3a after deprotection), and deoxyguanosine derivatives were demonstrated through metalation-electrophilic fluorination by the treatment of suitably protected purine nucleosides with lithium diisopropylamide (LDA) followed by N-fluorobenzenesulfonimide (NFSI) [14], all in low or moderate yields. In this chemistry, guanosine derivatives had to be protected at O-6 position. We found that N-2-isobutyryl protected, but not N-2-formamidine protected, 2′-deoxyguanosine, compounds 6 and 8, respectively, can be fluorinated using this chemistry. Thus, 3′,5′-O-bis(tert-butyldimethylsilyl)-N-2-isobutyryl-2′-deoxyguanosine 6 was subjected to metalation−electrophilic fluorination with LDA and NSFI to generate the corresponding 8-fluoro analog 7 in 30% yield (Scheme 1), with recovery of 50% the starting material 6. A similar reaction with 3′,5′-O-bis(tert-butyldimethylsilyl)-N-2- dimethylformamidine-2′-deoxyguanosine 8 failed to give the corresponding fluorinated product 9.
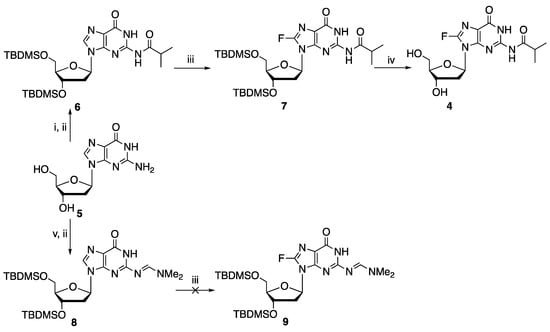
Scheme 1.
Synthesis of 8-fluoro-deoxyguanosine derivatives. Reagents and conditions: (i). a. (CH3)3SiCl, ((CH3)2CHCO)O, C5H5N, r.t. 2.5 h; b. aq. NH3, 0 °C, 30 min; (ii). TBDMSCl, DMF, r.t., 24 h; (iii). a. LDA, toluene, −78 °C, 2 h; b. NFSI; (iv). TBAF, THF, r.t., 1 h; (v). (CH3)2NCH(OCH3)2, MeOH, r.t., 2 d.
In order to introduce 8-fluoro-2′-deoxyguanosine into oligonucleotides through the phosphoramidite chemistry-based solid phase synthesis, the stability of 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine 12 in acidic conditions was investigated. This property is of importance as di- or trichloroacetic acid (DCA and TCA, respectively) is typically used for the removal of dimethoxytrityl (DMTr) group during the detritylation reaction. As such, 8-fluoro-2′-deoxyguanosine derivatives will need to be sufficiently stable under the acidic detritylation condition. Thus, experiments were conducted by 19F NMR spectroscopy to determine the stability of 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 in acids of different pKa.
As seen in Table 1, 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 is stable in 3% benzoic acid and 80% acetic acid in methanol, and relatively stable in monochloroacetic acid (MCA), however, removal of the DMTr group with these acids is reversible. While 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 is relatively unstable in DCA and TCA, It was previously shown that DMTr removal can be effected by DCA and TCA in as little as 20 s [15], thus, DCA appears to be the most suited for the removal of DMTr groups in solid phase synthesis involving 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4. This nucleoside was subsequently protected with DMTr at 5′-OH (as in 10) and then transformed into the corresponding 3′-phosphoramidite 11 (Scheme 2).

Table 1.
Half-lives of 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 under acidic conditions. (TCA: trichloroacetic acid; DCA: dichloroacetic acid; MCA: monochloroacetic acid).
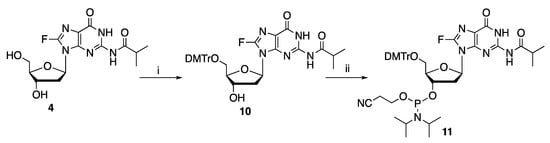
Scheme 2.
Synthesis of phosphoramidite 11. Reagents and conditions: (i). DMTr-Cl, C5H5N, r.t., 30 min; (ii). NCCH2CH2P(Cl)N(i-Pr)2, EtN(i-Pr)2, r.t., 30 min.
Solid phase syntheses were carried out using the ABI standard 1 μmol cycle conditions for the assembly of d(CG)6 sequences where a single dG was replaced with 8-fluoro-dG at each of the dG positions. The products were cleaved from the solid support and deprotected by incubation with concentrated aqueous ammonium hydroxide at 55 °C overnight. All modified sequences were found by mass spectrometry to contain a major species with a mass of 3753 Da, which is 87 mass unit higher than the expected sequences (3665 Da). The exact nature of the modification during solid phase synthesis that led to the formation of this higher mass species remains to be identified. It was found that the treatment of 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine 12 with the activator (5-ethylthio-1H-tetrazole) for coupling reactions or the oxidation solution containing aqueous iodine in pyridine, did not lead to the formation of displacement products 13 and 14, respectively, which, if reacted with 2,6-lutidine during the capping step, could generate the corresponding modified dG species 15 with the 87 extra mass units found in the oligonucleotides (Figure 2).
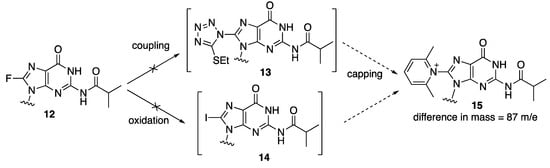
Figure 2.
Hypothesized formation of a 2,6-lutidine–guanine adduct 15 during solid phase synthesis.
Finally, it was found that treatment of 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 (Scheme 3) with concentrated aqueous ammonium hydroxide at 55 °C overnight, the typical conditions for the deprotection of N-acyl groups, did not give the desired 8-fluoro-2′-deoxyguanosine 18; instead, a mixture of 8-amino-2′-deoxyguanosine 16 and C8:5′-O-cyclo-2′-deoxyguanosine 17 was obtained in approximately a 2:1 ratio, as indicated by the reverse phase HPLC profile in Figure S28.
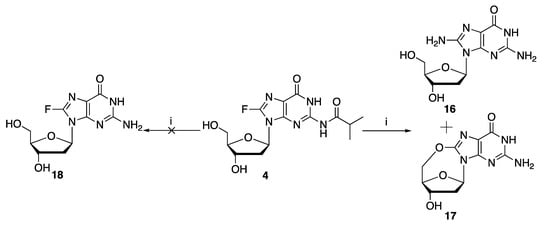
Scheme 3.
Attempted deprotection of 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4. (i). conc. aq. NH4OH, 55 °C, overnight.
Taken together, these results suggest that 8-fluoro-N-isobutyryl-2′-deoxyguanosine 4 is unsuitable for the introduction of 8-fluoro-2′-deoxyguanosine into oligonucleotides via the phosphoramidite chemistry-based solid phase synthesis. Alternative N2-protecting groups, as well as modified solid phase synthesis conditions, will be required for successful incorporation of 8-fluoro-2′-deoxyguanosine into oligonucleotides.
3. Materials and Methods
1H NMR spectra were measured at 400 MHz with a Bruker Avance 400 Digital NMR spectrometer (Billerica, MA, USA). 13C, 31P and 19F NMR spectra were recorded at 100.6, 162.0 and 376.6 MHz respectively, with the same spectrometer. Chemical shifts and coupling constants (J values) are given in ppm and Hz, respectively. Deuterated solvents were purchased from C/D/N Isotopes (Montreal, QC, Canada). EI (electron impact) and FAB (fast atom bombardment) mass spectra were obtained with a Thermo Scientific DFS mass spectrometer (Waltham, MA, USA); ESI (electrospray) spectra were measured with a Bruker HCT Plus ion-trap mass spectrometer. Desican silica gel (230–400 mesh) was used for column chromatography. Thin layer chromatography was performed on Silicycle F-254 silica TLC plates using the following solvent mixtures:
Solvent A: methanol-dichloromethane (5:95, v/v)
Solvent B: methanol-dichloromethane (20:80, v/v)
3.1.Solvents and Chemicals
Toluene was dried by heating under reflux over sodium in the presence of benzophenone for 4 h and then distilled under nitrogen. N,N-Diisopropylethylamine and pyridine were dried by heating under reflux over calcium hydride for 4 h and then distilled under nitrogen. N,N-Dimethylformamide was dried by heating at 60 °C over calcium hydride for 4 h and then distilled under vacuum. Dichloromethane was dried by heating under reflux over phosphorus pentoxide for 4 h and then distilled under nitrogen. All other reagents were purchased from Sigma-Aldrich or TCI America without further purification prior to use unless stated otherwise.
3.2. 3′,5′-Bis(tert-butyldimethylsilyl)-N-isobutyryl-2′-deoxyguanosine (6)
N-Isobutyryl-2′-deoxyguanosine 5 (3.50 g, 10.4 mmol), imidazole (4.66 g, 68.6 mmol) and tert-butyldimethylsilyl chloride (6.90 g, 45.7 mmol) were stirred in dry dimethylformamide (30 mL) for 5 h. The resulting slurry was diluted with ethanol (25 mL) and stored at −20 °C for 24 h. White crystals were collected by vacuum filtration to give the title compound (4.93 g, 84%). M.p. 102–104 °C (ethanol). Rf (system A): 0.38. HR-MS (EI) found 565.3099, C26H47N5O5Si2 required 565.3116. δH (CDCl3): 0.09, 0.10, 0.117 and 0.119 (total 12 H, Si(CH3)2), 0.920 and 0.925 (total 18 H, Si(C(CH3)3), 1.27 (3 H, d, J = 6.5, iBu), 1.29 (3 H, d, J = 6.5), 2.37 (1 H, ddd, J = 3.8, 6.0 and 10.0, H-2′), 2.47 (1 H, m, H-2′’), 2.65 (1 H, CH(CH3)2, septet, J = 6.9), 3.78 (2 H, m, H-5′/5′’), 3.99 (1 H, q, J = 3.4, H-4′), 4.58 (1 H, m, H-3′), 6.23 (1 H, dd, J = 6.5 and 6.5, H-1′), 7.98 (1 H, s, H-8), 8.40 (1 H, s, NH), 12.00 (1 H, s, NH). δC (CDCl3): −5.51, −5.38, −4.77, −4.68, 18.0, 18.4, 18.95, 19.01, 25.7, 26.0, 36.6, 41.4, 62.8, 71.8, 83.5, 88.0, 121.5, 136.8, 147.3, 147.8, 155.5, 178.1.
3.3. 3′,5′-Bis(tert-butyldimethylsilyl)-8-fluoro-N-isobutyryl-2′-deoxyguanosine (7)
3′,5′-Bis(tert-butyldimethylsilyl)-N-isobutyryl-2′-deoxyguanosine 6 (4.00 g, 7.08 mmol) was co-evaporated with dry toluene (3 × 15 mL) and dissolved in dry toluene (80 mL). After the mixture was cooled to −78 °C (acetone–dry ice bath), 2.0 M lithium diisopropylamide solution in THF/heptane/ethylbenzene (17.7 mL, 35.4 mmol) was added slowly. After 2 h, N-fluorobenzenesulphonamide (6.68 g, 21.2 mmol) was added and the mixture was stirred for 1.5 h at −78 °C and then allowed to warm up to room temperature over 30 min. Saturated aqueous ammonium chloride solution (80 mL) was added and the layers were separated. The aqueous layer was extracted with dichloromethane (2 × 40 mL), and the combined organic layers were washed with saturated sodium bicarbonate solution (100 mL), dried over magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel. The appropriate fractions, which were eluted with dichloromethane–methanol (99.3:0.7 v/v), were pooled and concentrated under reduced pressure to give the title compound as a pale yellow solid (825 mg, 20%). Rf (system A): 0.41. HR-MS (EI) found 583.3015, C26H46N5O5Si2F required 583.3022. δH (CDCl3): 0.04 (3 H, s, Si(CH3)2), 0.06 (3 H, s, Si(CH3)2), 0.13 (6 H, s, Si(CH3)2), 0.89 (9 H, s, Si(C(CH3)3)2), 0.93 (9 H, s, Si(C(CH3)3)2), 1.24 (3 H, d, J = 7.0, CH(CH3)2), 1.27 (3 H, d, J = 7.0, CH(CH3)2), 2.26 (1 H, ddd, J = 3.9, 6.6 and 10.7, H-2′), 2.66 (1 H, CH(CH3)2, septet, J = 6.9), 2.78 (1 H, m, H-2′’), 3.71 (2 H, m, H-5′/5′’), 3.89 (1 H, m, H-4′), 4.59 (1 H, m, H-3′), 6.16 (1 H, dd, J = 6.8 and 6.8, H-1′), 8.69 (1 H, s, NH), 12.12 (1 H, s, NH). δF (CDCl3): −101.3. δC (CDCl3): −5.5, −5.4, −4.8, −4.7, 18.0, 18.4, 18.8, 19.1, 25.8, 25.9, 36.5, 37.9, 62.7, 71.7, 82.3, 87.7, 114.8 (d, JC,F = 13.9), 146.7 (d, JC,F = 3.3), 147.7 (d, JC,F = 2.7), 149.6 (d, JC,F = 251.1), 154.8, 178.5.
3.4. 8-Fluoro-N-isobutyryl-2′-deoxyguanosine (4)
3′,5′-Bis(tert-butyldimethylsilyl)-8-fluoro-N-isobutyryl-2′-deoxyguanosine 7 (350 mg, 0.60 mmol) and tetra-n-butylammonium fluoride (391 mg, 1.5 mmol) were stirred in tetrahydrofuran (12 mL) for 1 h at r.t., followed by addition of warm water (50 °C) until all salts dissolved. The product crystalized from water to give 127 mg (60%) of the title compound as white crystals. M.p.: decomposed at 183–185 °C (water). Rf (system B): 0.72. HR-MS (EI) found 335.1220, C14H18N5O5F requires 355.1292. δH (DMSO-d6): 1.13 (6 H, d, J = 6.6, CH(CH3)2), 2.24 (1 H, ddd, J = 3.8, 6.5 and 10.2, H-2′), 2.76 (2 H, m, H-2′’ and CH(CH3)2), 3.79 (1 H, m, H-4′), 4.37 (1 H, m, H-3′), 4.84 (1 H, t, J = 5.6, 5′-OH, ex), 5.35 (1 H, d, J = 4.0, 3′-OH, ex), 6.17 (1 H, t, J = 6.9, H-1′), 11.68 (1 H, s, NH, ex), 12.17 (1 H, s, NH, ex). δC (DMSO-d6): 14.0, 19.3, 35.3, 37.5, 62.0, 70.7, 82.8, 88.2, 113.9 (d, JC,F = 13.1), 147.5 (d, JC,F = 3.2), 148.9 (d, JC,F = 2.6), 149.5 (d, JC,F = 247.6), 154.4 (d, JC,F = 2.1), 180.6. δF (DMSO-d6): −102.9.
3.5. 5′-(4,4′-Dimethoxytrityl)-8-fluoro-N-isobutyryl-2′-deoxyguanosine (10)
8-Fluoro-N-isobutyryl-2′-deoxyguanosine 4 (0.50 g, 1.4 mmol) was co-evaporated with dry pyridine (3 × 10 mL) and dissolved in dry pyridine (5 mL), followed by addition of 4,4′-dimethoxytrityl chloride (0.72 g, 2.1 mmol). After 30 min, the reaction was quenched with a triethylamine/ethanol (1:1 v/v) mixture (1 mL). The products were diluted with dichloromethane (50 mL) and extracted with saturated aqueous sodium hydrogen carbonate (50 mL). The aqueous layer was back-extracted with dichloromethane (30 mL) and the combined organic layers were dried (MgSO4) and evaporated under reduced pressure. The oily residue was purified by column chromatography on silica gel. The appropriate fractions, which were eluted with dichloromethane–ethanol–triethylamine (98.2:1.5:0.3 v/v/v), were pooled and concentrated under reduced pressure to give the title compound as a colorless glass (850 mg, 92%). Rf (system A): 0.27. MS (ESI) found 658.2 (M-H+), C35H37FN5O7+ required 658.2677. δH (CDCl3): 0.98 (3 H, d, J = 6.7), 1.07 (3 H, d, J = 7.0), 2.27 (1 H, CH(CH3)2, m), 2.34 (1 H, ddd, J = 4.2, 6.2 and 10.6, H-2′), 3.00 (1 H, m, H-2′’), 3.24 (1 H, dd, J = 5.2 and 10.3, H-5′), 3.36 (1 H, dd, J = 3.7 and 10.3, H-5′’), 3.74 and 3.75 (6 H, (OCH3)2), 4.09 (1 H, ddd, J = 4.1, 4.1 and 8.5, H-4′), 4.82 (1 H, m, H-3′), 6.14 (1 H, dd, J = 6.2 and 6.2, H-1′), 6.74 (3 H, d, J = 3.1), 6.77 (3 H, d, J = 3.1), 7.14–7.23 (3 H, m), 7.30 and 7.32 (4 H), 7.44 (2 H, dd, J = 1.5 and 8.3). δF(CDCl3): −103.7. δC (CDCl3): 18.75, 18.77, 36.0, 36.2, 55.2, 63.9, 71.4, 82.8, 86.1, 86.3, 113.1, 115.0, 126.9, 127.8, 128.1, 129.96, 130.0, 135.9, 136.1, 144.9, 146.9, 147.8, 148.6, 158.5, 179.4.
3.6. 5′-(4,4′-Dimethoxytrityl)-8-fluoro-N-isobutyryl-2′-deoxyguanosine-3′-(2-cyanoethyl)-N,N-diisopropylphosphoramidite (11)
5′-(4,4′-Dimethoxytrityl)-8-fluoro-N-isobutyryl-2′-deoxyguanosine 10 (1.00 g, 1.52 mmol) was co-evaporated with dry toluene (3 × 10 mL) and then dissolved in dry dichloromethane (20 mL), followed by addition of dry N,N-diisopropylethylamine (1.0 mL, 5.7 mmol) and (2-cyanoethyl)-N,N-diisopropyl phosphochloridite (0.72 g, 3.0 mmol). After the reaction mixture was stirred for 30 min, triethylamine (1 mL) was added and products were concentrated under reduced pressure. The residue was purified by column chromatography on silica gel. The appropriate fractions, which were eluted with hexane–acetone–triethylamine (70:28:2 v/v/v), were pooled and concentrated under reduced pressure. The resulting oil was dissolved in dichloromethane (2 mL) and added dropwise to pentane (20 mL) at −40 °C under stirring. The cloudy suspension was centrifuged for 20 min and the supernatant was decanted. The white solid was collected and dried to give the title phosphoramidite (0.57 g, 44%). HR-MS (FAB) found 858.3787 ([M + H]+), C44H54FN7O8P+ required 858.3755. δH(CDCl3) include the following signals: 2.45–2.56 (1 H, m, H-2′), 3.12–3.51 (3 H, m, H-2”, H-5′, and H-5”), 3.774, 3.777, 3.782 and 3.787 (6 H, -OMe), 4.21–4.29 (1 H, m, H-4′), 4.77–4.89 (1 H, m, H-3′), 6.19 (1 H, m, H-1′), 6.75–6.79 (4 H, m). δF (CDCl3): −106.27 and −104.28. δP(CDCl3): 147.78 and 147.96.
3.7. Treatment of 8-Fluoro-N-isobutyryl-2′-deoxyguanosine under Ammonolysis Conditions
8-Fluoro-N-isobutyryl-2′-deoxyguanosine (4 mg) was incubated with concentrated aqueous ammonium hydroxide (1.0 mL) at 55 °C for 16 h. Upon cooling the products were lyophilized. The residue was purified by column chromatography using C18-reverse phase matrix (1 × 15 cm) on a BioRad DuoFlow FPLC system. The column was eluted with acetonitrile–water (0:100 to 30:70 v/v) in a linear gradient over 60 min. Eluants were collected in 1.0 mL fractions and lyophilized.
3.8. Oligonucleotide Synthesis
Oligonucleotides were synthesized using an ABI 3400 DNA synthesizer (Waltham, MA, USA) under the standard 1 µmol cycle conditions. Phosphoramidites were prepared as 100 mM solutions in dry acetonitrile. A solution of 5-(ethylthio)-1H-tetrazole (250 mM in dry acetonitrile) was used as the activator, and coupling reaction time was set at 60 s. Detritylation was effected by delivering dichloroacetic acid (3% in dichloromethane) to reaction columns for 110 s. After solid phase synthesis was complete, the products were cleaved from solid support and deprotected by incubation with ammonium hydroxide at 55 °C for 24 h and then lyophilized.
4. Conclusions
3′,5′-O-Bis(tert-butyldimethylsilyl)-8-fluoro-N-2-isobutyryl-2′-deoxyguanosine was synthesized by treating 3′,5′-O-bis(tert-butyldimethylsilyl)-N-2-isobutyryl-2′-deoxyguanosine with LDA and N-fluorobenzenesulfonimide. Subsequent deprotection afforded 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine. The latter compound was found to be sufficiently stable in 3% dichloroacetic acid, however, incorporation of the corresponding phosphoramidite into d(CG)6 sequences did not lead to the formation of the desired 8-fluoro-2′-deoxyguanosine modified oligonucleotides. Treatment of 8-fluoro-N-2-isobutyryl-2′-deoxyguanosine under ammonolysis conditions led to the formation of a mixture of 8-amino-2′-deoxyguanosine and C8:5′-O-cyclo-2′-deoxyguanosine. Future work will explore protecting groups for the exocyclic amine that are readily removable under conditions that do not lead to modification of this nucleoside. Furthermore, cycle conditions for solid phase synthesis need to be modified in order to successfully incorporate 8-fluoro-2′-deoxyguanosine.
Supplementary Materials
The supplementary materials are available online.
Author Contributions
Conceptualization, A.S. and H.Y.; methodology, A.S., J.H., S.O. and H.Y.; experiment, A.S., J.H. and S.O.; writing, review and editing, A.S., J.H., S.O. and H.Y.; supervision, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Guo, F.; Li, Q.; Zhou, C. Synthesis and biological applications of fluoro-modified nucleic acids. Org. Biomol. Chem. 2017, 15, 9552–9565. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Viel, S.; Ziarelli, F.; Peng, L. 19F NMR: A valuable tool for studying biological events. Chem. Soc. Rev. 2013, 42, 7971–7982. [Google Scholar] [CrossRef] [PubMed]
- Solodinin, A.; Gautrais, A.; Ollivier, S.; Yan, H. 5-Fluoro-2′-deoxycytidine as a probe for the study of B/Z-DNA transition by 19F NMR spectroscopy. Acs Omega 2019, 4, 19716–19722. [Google Scholar] [CrossRef]
- Ye, S.; Rezende, M.M.; Deng, W.P.; Kirk, K.L. Convenient synthesis of 2′-deoxy-2-fluoroadenosine from 2-fluoroadenine. NucleosidesNucleotides Nucleic Acids 2003, 22, 1899–1905. [Google Scholar] [CrossRef]
- Nóbile, M.; Terreni, M.; Lewkowicz, E.; Iribarren, A.M. Aeromonas hydrophila strains as biocatalysts for transglycosylation. Biocatal. Biotransform. 2010, 28, 395–402. [Google Scholar] [CrossRef]
- Zhou, X.; Szeker, K.; Jiao, L.-Y.; Oestreich, M.; Mikhailopulo, I.A.; Neubauer, P. Synthesis of 2,6-dihalogenated purine nucleosides by thermostable nucleoside phosphorylases. Adv. Synth. Catal. 2015, 357, 1237–1244. [Google Scholar] [CrossRef]
- Pérez, E.; Sánchez-Murcia, P.A.; Jordaan, J.; Blanco, M.D.; Mancheño, J.M.; Gago, F.; Fernández-Lucas, J. Enzymatic synthesis of therapeutic nucleosides using a highly versatile purine nucleoside 2′-deoxyribosyl transferase from Trypanosoma brucei. ChemCatChem 2018, 10, 4406–4416. [Google Scholar] [CrossRef]
- Krolikiewicz, K.; Vorbrüggen, H. The synthesis of 2-fluoropurine nucleosides. Nucleosides Nucleotides 1994, 13, 673–678. [Google Scholar] [CrossRef]
- Ikehara, M.; Yamada, S. Studies of nucleosides and nucleotides. XLIX. Synthesis of 8-fluoroadenosine. Chem. Pharm. Bull. 1971, 19, 104–109. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, Y.; Kumadaki, I.; Ohsawa, A.; Murakami, S.-I. Synthesis of 2′,3′,5′-tris-O-acetyl-8-fluoroadenosine. J. Chem. Soc., Chem. Commun. 1976, 12, 430–431. [Google Scholar] [CrossRef]
- Barrio, J.R.; Namavari, M.; Keen, R.E.; Satyamurthy, N. The elusive 8-fluoroadenosine. Tetrahedron Lett. 1998, 39, 7231–7234. [Google Scholar] [CrossRef]
- Butora, G.; Schmitt, C.; Levorse, D.A.; Streckfuss, E.; Doss, G.A.; MacCoss, M. The elusive 8-fluoroadenosine: A simple non-enzymatic synthesis and characterization. Tetrahedron 2007, 63, 3782–3789. [Google Scholar] [CrossRef]
- Barrio, J.R.; Namavari, M.; Phelps, M.E.; Satyamurthy, N. Regioselective fluorination of substituted guanines with dilute F2: A facile entry to 8-fluoroguanine derivatives. J. Org. Chem. 1996, 61, 6084–6085. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Lagisetty, P.; Zajc, B. Direct synthesis of 8-fluoro purine nucleosides via metalation−fluorination. J. Org. Chem. 2007, 72, 8222–8226. [Google Scholar] [CrossRef] [PubMed]
- Tram, K.; Sangvi, Y.; Yan, H. Further studies on the detritylation in solid phase oligodeoxyribonucleotide synthesis. Nucleoside Nucleotides Nucleic Acids 2011, 30, 12–19. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).