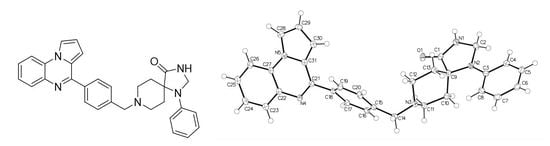
1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity
Abstract
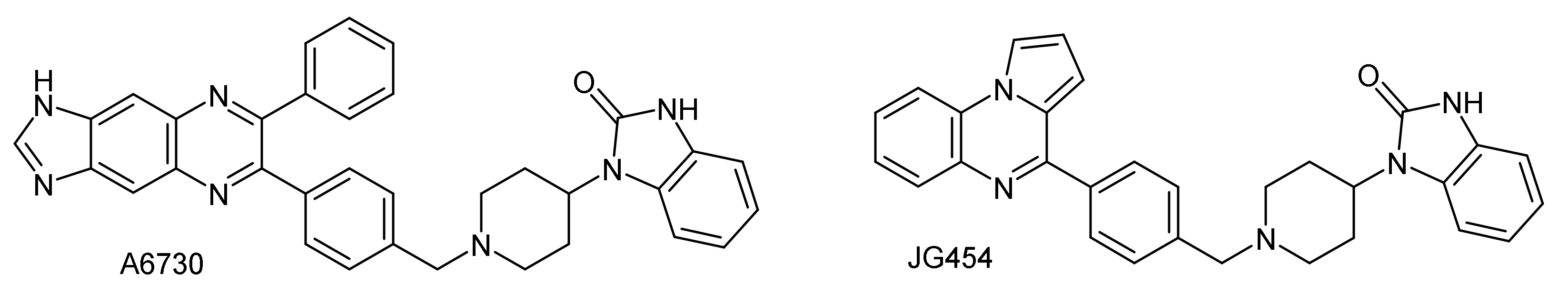
1. Introduction
2. Results and Discussion
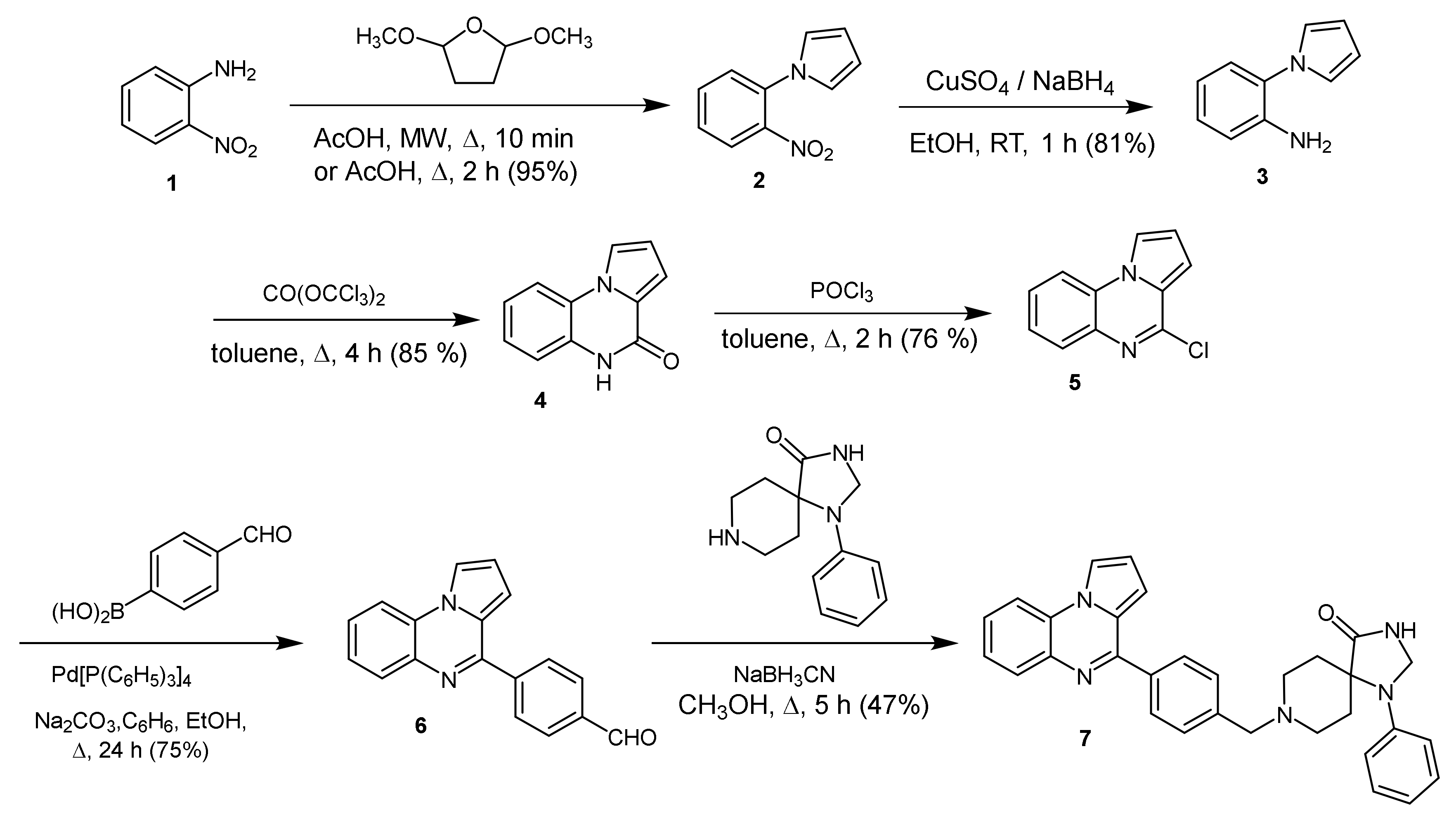
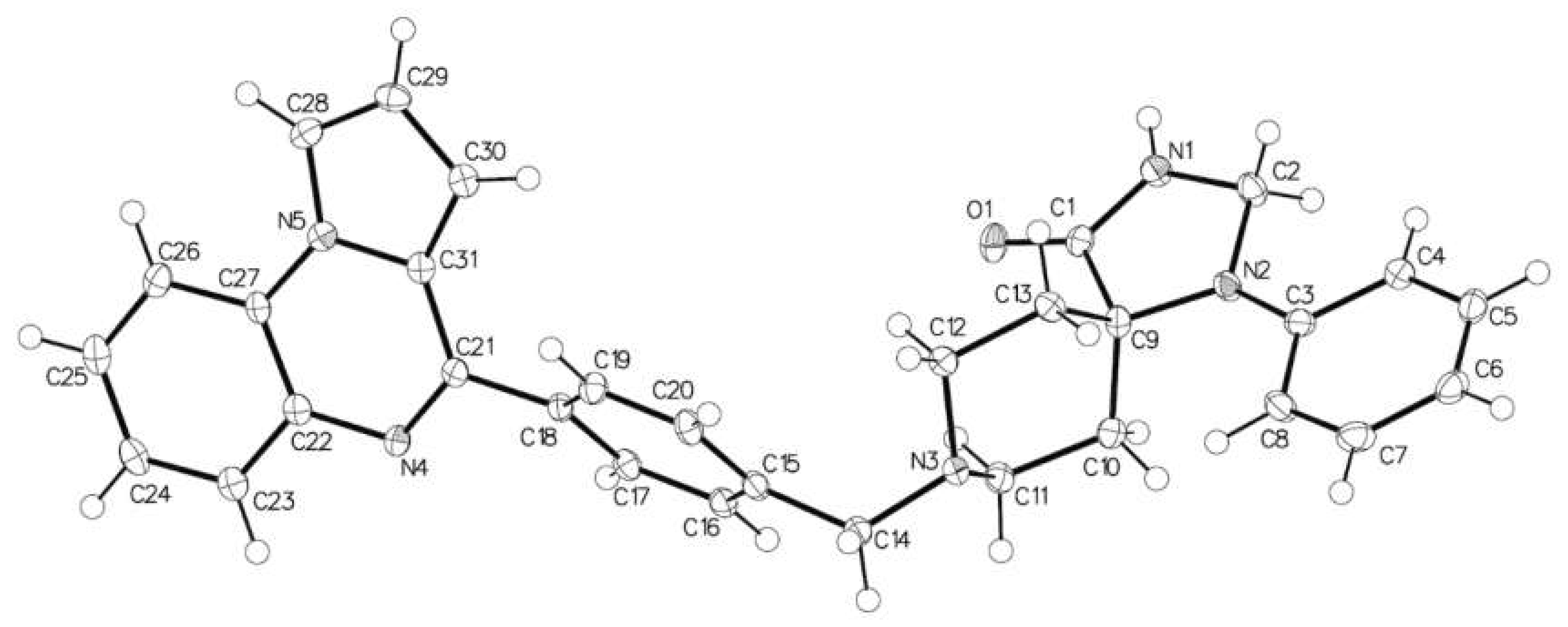
2.1. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one
2.2. Cytotoxic Activity
3. Materials and Methods
3.1. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one (7)
3.2. X-ray Data
3.3. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, A.; Ma, C. Recent progress in biological activities and synthetic methodologies of pyrroloquinoxalines. Mini-Rev. Med. Chem. 2013, 13, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M. New achievements in the synthesis of pyrrolo[1,2-a]quinoxaline. Chem. Heterocycl. Compd. 2019, 55, 584–597. [Google Scholar] [CrossRef]
- Campiani, G.; Butini, S.; Fattorusso, C.; Trotta, F.; Franceschina, S.; De Angelis, M.; Nielsen, K.S. Novel aryl piperazine derivatives with medical utility. U.S. Patent 11/794,687, 24 September 2006. [Google Scholar]
- Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armaroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; et al. Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J. Med. Chem. 2001, 44, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Schann, S.; Mayer, S.; Gardan, S. Pyrrolo[1,2-a]quinoxaline derivatives as Adenosine A3 receptor modulators and uses thereof. U.S. Patent 12/158,035, 28 June 2007. [Google Scholar]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.-M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, antimalarial activity and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; et al. Synthesis, Analytical Behaviour and Biological Evaluation of New 4-Substituted Pyrrolo[1,2-a]quinoxalines as Antileishmanial Agents. Bioorg. Med. Chem. 2007, 15, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Moreau, S.; Ronga, L.; Basmaciyan, L.; Cohen, A.; Rubio, S.; Bentzinger, G.; Azas, N.; Mullié, C.; Sonnet, P. Design, Synthesis and Antimalarial Activity of Some New Aminoalcohol-pyrrolo[1,2-a]quinoxaline Derivatives. Lett. Drug Des. Discovery 2016, 13, 932–942. [Google Scholar] [CrossRef]
- Guillon, J.; Moreau, S.; Mouray, E.; Sinou, V.; Forfar, I.; Belisle-Fabre, S.; Desplat, V.; Millet, P.; Parzy, D.; Jarry, C.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and In Vitro Antimalarial Activity. Bioorg. Med. Chem. 2008, 16, 9133–9144. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Mouray, E.; Moreau, S.; Mullié, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Ravanello, F.; Le-Naour, A.; Pinaud, N.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and in Vitro Antimalarial Activity—Part II. Eur. J. Med. Chem. 2011, 46, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le Pape, P.; Savrimoutou, S.; Pinaud, N.; Mullié, C.; Daulouede, S.; Vincendeau, P.; et al. Design, Synthesis and Biological Evaluation of Novel 4-Alkapolyenylpyrrolo[1,2-a]quinoxalines as Antileishmanial Agents—Part III. Eur. J. Med. Chem. 2014, 81, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S. Synthesis and evaluation of the cytotoxic activity of novel ethyl 4-[4-(4-substitutedpiperidin-1-yl)]benzyl-phenylpyrrolo[1,2-a]quinoxaline-carboxylate derivatives in myeloid and lymphoid leukemia cell lines. Eur. J. Med. Chem. 2016, 113, 214–227. [Google Scholar] [PubMed]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; et al. Synthesis and Antiproliferative Effect of Ethyl 4-[4-(4-Substituted Piperidin-1-yl)]benzylpyrrolo[1,2-a]quinoxalinecarboxylate Derivatives on Human Leukemia Cells. ChemMedChem 2017, 12, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Desplat, V.; Geneste, A.; Begorre, M.-A.; Belisle-Fabre, S.; Brajot, S.; Massip, S.; Thiolat, D.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis of New Pyrrolo[1,2-a]quinoxaline Derivatives as Potential Inhibitors of Akt Kinase. J. Enzyme Inhib. Med. Chem. 2008, 23, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Desplat Moreau, S.; Gay, A.; Belisle-Fabre, S.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis and evaluation of the antiproliferative ctivity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt Kinase. Part II. J. Enzyme Inhib. Med. Chem. 2010, 25, 204–215. [Google Scholar]
- Molina, P.; Alajarin, M.; Vidal, A. New methodology for the preparation of pyrrole and indole derivatives via iminophosphorane: Synthesis of pyrrolo[l,2-a]quinoxalines, indolo[3,2-c]quinolines and indolo[l,2-c]quinazolines. Tetrahedron 1990, 46, 1063–1078. [Google Scholar] [CrossRef]
- Campiani, G.; Nacci, V.; Corelli, F.; Anzini, M. Polycondensed Heterocycles. VII. A Convenient Synthesis of Pyrrolo[1,2-a]quinoxaline Derivatives by Intramolecular Aromatic Nucleophilic Displacement. Synth. Comm. 1991, 21, 1567–1576. [Google Scholar] [CrossRef]
- Yuan, Q.; Ma, D. A one-pot coupling/Hydrolysis/Condensation process to Pyrrolo[1,2-a]quinoxaline. J. Org. Chem. 2008, 73, 5159–5162. [Google Scholar] [CrossRef] [PubMed]
- Supplementary X-ray crystallographic data: Cambridge Crystallographic Data Centre, University Chemical Lab, Lensfield Road, Cambridge, CB2 1EW, UK. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 24 January 2020).
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Desplat, V. 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Molbank 2018, 2018, M1023. [Google Scholar] [CrossRef]
| Compound | IC50 Values (μM) (a) | |||
|---|---|---|---|---|
| K562 | HL60 | U937 | PBMNC + PHA | |
| 7 | 3.5 ± 0.2 | 15.0 ± 0.4 | >20 | 35.0 ± 0.5 |
| JG454 | 4.5 ± 0.2 | 14.0 ± 0.4 | >20 | 10.0 ± 0.5 |
| A6730 | 17.0 ± 0.3 | 5.5 ± 0.2 | 8.0 ± 0.2 | >50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillon, J.; Savrimoutou, S.; Rubio, S.; Moreau, S.; Pinaud, N.; Marchivie, M.; Desplat, V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity. Molbank 2020, 2020, M1113. https://doi.org/10.3390/M1113
Guillon J, Savrimoutou S, Rubio S, Moreau S, Pinaud N, Marchivie M, Desplat V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity. Molbank. 2020; 2020(1):M1113. https://doi.org/10.3390/M1113
Chicago/Turabian StyleGuillon, Jean, Solène Savrimoutou, Sandra Rubio, Stéphane Moreau, Noël Pinaud, Mathieu Marchivie, and Vanessa Desplat. 2020. "1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity" Molbank 2020, no. 1: M1113. https://doi.org/10.3390/M1113
APA StyleGuillon, J., Savrimoutou, S., Rubio, S., Moreau, S., Pinaud, N., Marchivie, M., & Desplat, V. (2020). 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-Leukemic Activity. Molbank, 2020(1), M1113. https://doi.org/10.3390/M1113