N′-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide
Abstract
1. Introduction
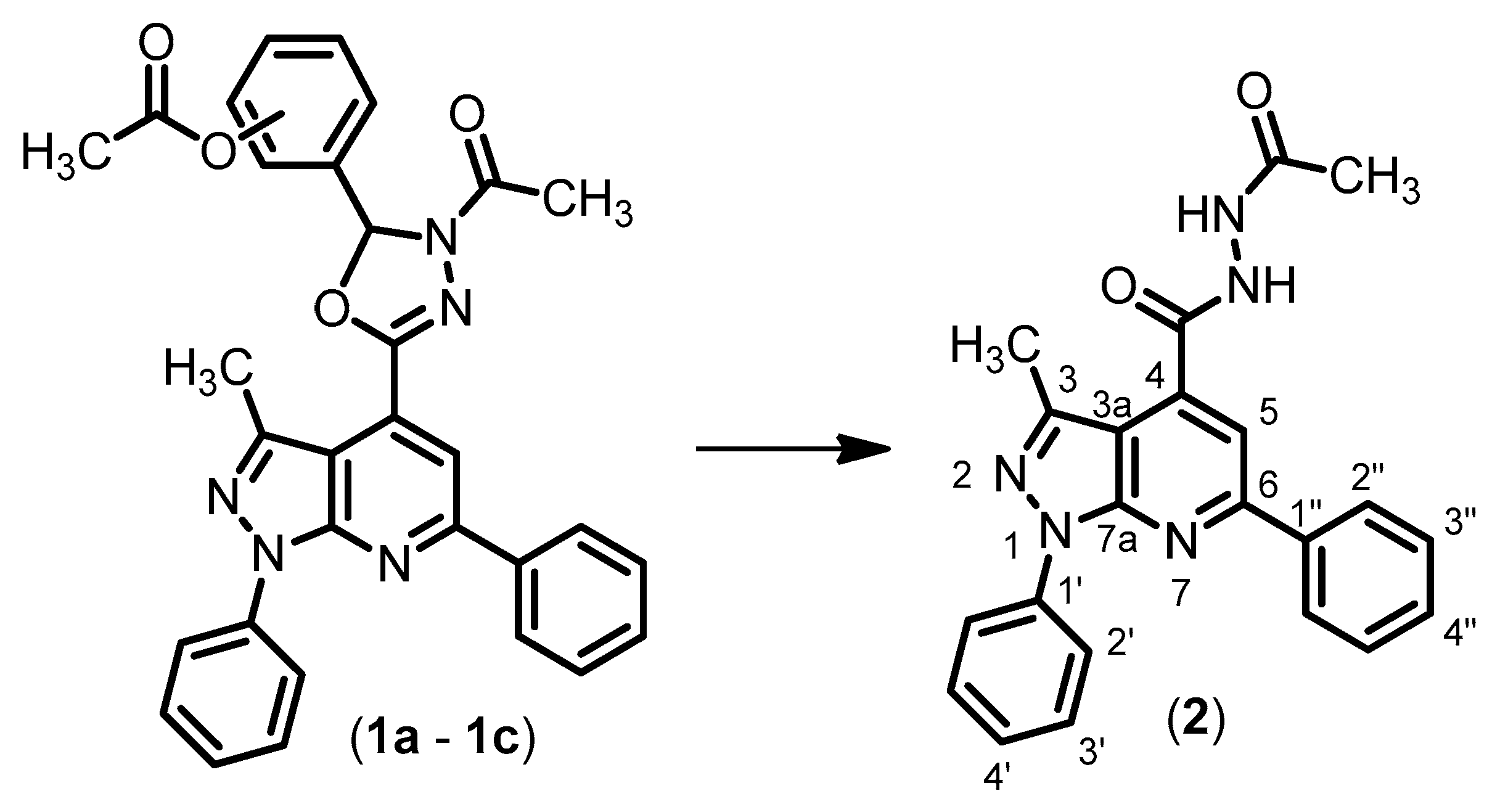
2. Results and Discussion
3. Materials and Methods
N’-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide (2)
Method A
Method B
Method C
Structural Characterization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azab, M.; El-Hashash, M.; Morsy, J.; Mahmoud, N. Design, synthesis and anticancer activity of novel 2,3- and 2,4-disubstituted quinazoline and quinazolinone derivatives. Heterocycles 2016, 92, 316. [Google Scholar] [CrossRef]
- Joshi, S.D.; Dixit, S.R.; Kulkarni, V.H.; Lherbet, C.; Nadagouda, M.N.; Aminabhavi, T.M. Synthesis, biological evaluation and in silico molecular modeling of pyrrolyl benzohydrazide derivatives as enoyl ACP reductase inhibitors. Eur. J. Med. Chem. 2017, 126, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, M.F.; Angers, L.; Hamel, J.D.; Paquin, J.F. Synthesis of 1,3,4-oxadiazoles from 1,2-diacylhydrazines using [Et 2NSF 2]BF 4 as a practical cyclodehydration agent. Org. Biomol. Chem. 2012, 10, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Ali, B.; Khan, K.M.; Iqbal, J.; Ur Rahman, S.; Zaib, S.; Perveen, S. Synthesis and in vitro urease inhibitory activity of benzohydrazide derivatives, in silico and kinetic studies. Bioorg. Chem. 2019, 82, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in medicinal chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Khalilullah, H.; Ahsan, M.J.; Hedaitullah, M.; Khan, S.; Ahmed, B. 1,3,4-oxadiazole: A biologically active scaffold. Mini Rev. Med. Chem. 2012, 12, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Pitasse-Santos, P.; Sueth-Santiago, V.; Lima, M.E.F. 1,2,4- and 1,3,4-oxadiazoles as scaffolds in the development of antiparasitic agents. J. Braz. Chem. Soc. 2018, 29, 435–456. [Google Scholar] [CrossRef]
- Preziosi, P. Isoniazid: Metabolic aspects and toxicological correlates. Curr. Drug Metab. 2007, 8, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Semina, I.I.; Balashov, V.P.; Kurmysheva, T.V.; Tarasova, R.I.; Shilovskaya, E.V.; Voskresenskaya, O.V.; Baichurina, A.Z.; Al’Myasheva, M.I. Synthesis and pharmacological activity of (2-chloroethoxy-4′-dimethylaminophenyl)phosphorylacetic acid hydrazide (CAPAH) and its metabolite (N-acetyl derivative). Pharm. Chem. J. 2013, 47, 28–30. [Google Scholar] [CrossRef]
- Wang, G.B.; Wang, L.F.; Li, C.Z.; Sun, J.; Zhou, G.M.; Yang, D.C. A facile and efficient method for the selective deacylation of N-arylacetamides and 2-chloro-Narylacetamides catalyzed by SOCl2. Res. Chem. Intermed. 2012, 38, 77–89. [Google Scholar] [CrossRef]
- Soares, J.C.A.V. Planejamento, Síntese e Avaliação da Atividade Antichagásica de Novos Derivados 1H-Pirazolo[3,4-b]piridina. Master’s Thesis, Universidade Federal Fluminense, Niterói, Brazil, 2018. [Google Scholar]
- Narender, T.; Reddy, K.P.; Madhur, G. NaOAc-mediated selective deprotection of aromatic acetates and its application in the synthesis of natural products. Synth. Commun. 2009, 39, 1949–1956. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Di, F.; Xiong, L.; Yang, N.; Li, Y.; Li, Z. Synthesis and biological activities of 2,3-dihydro-1,3,4-oxadiazole compounds and its derivatives as potential activator of ryanodine receptors. Bioorg. Med. Chem. Lett. 2014, 24, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.H.; El-Fattah, B.A. Synthesis of certain pyridyl 1,3,4-oxadiazoles of biological interest and study of the cleavage of certain substituted oxadiazole rings with primary amines [abstract]. Egypt J. Pharm. Sci. 1985, 24, 49–56. [Google Scholar]
- Hamzi, I.; Fray, M.; Abidi, R.; Barhoumi-Slimi, T. Synthesis, characterization and conformational study of new α,β-unsaturated acylhydrazones based on calix [4]arene backbone. J. Mol. Struct. 2019, 1185, 78–84. [Google Scholar] [CrossRef]
- Litvinov, I.A.; Lodochnikova, O.A.; Bukharov, S.V.; Nugumanova, G.N.; Tagasheva, R.G.; Karamov, F.A. Molecular and crystal structure of indole and camfora with sterically hindered phenol fragments. J. Struct. Chem. 2019, 60, 308–314. [Google Scholar] [CrossRef]
- Lee, L.; Robb, L.M.; Lee, M.; Davis, R.; Mackay, H.; Chavda, S.; Babu, B.; O’Brien, E.L.; Risinger, A.L.; Mooberry, S.L.; et al. Design, synthesis, and biological evaluations of 2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoline analogs of combretastatin-A4. J. Med. Chem. 2010, 53, 325–334. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, J.C.A.V.; Dias, L.R.S. N′-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide. Molbank 2020, 2020, M1104. https://doi.org/10.3390/M1104
Soares JCAV, Dias LRS. N′-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide. Molbank. 2020; 2020(1):M1104. https://doi.org/10.3390/M1104
Chicago/Turabian StyleSoares, Júlio C. A. V., and Luiza R. S. Dias. 2020. "N′-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide" Molbank 2020, no. 1: M1104. https://doi.org/10.3390/M1104
APA StyleSoares, J. C. A. V., & Dias, L. R. S. (2020). N′-Acetyl-3-methyl-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-4-carbohydrazide. Molbank, 2020(1), M1104. https://doi.org/10.3390/M1104




