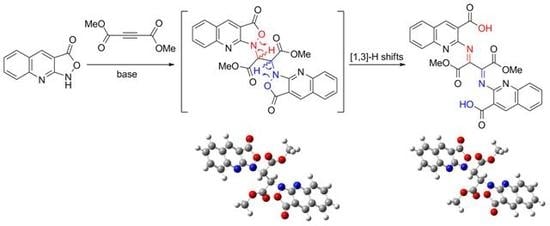
2,2′-((1,4-Dimethoxy-1,4-dioxobutane-2,3-diylidene)bis(azanylylidene))bis(quinoline-3-carboxylic acid)
Abstract
1. Introduction
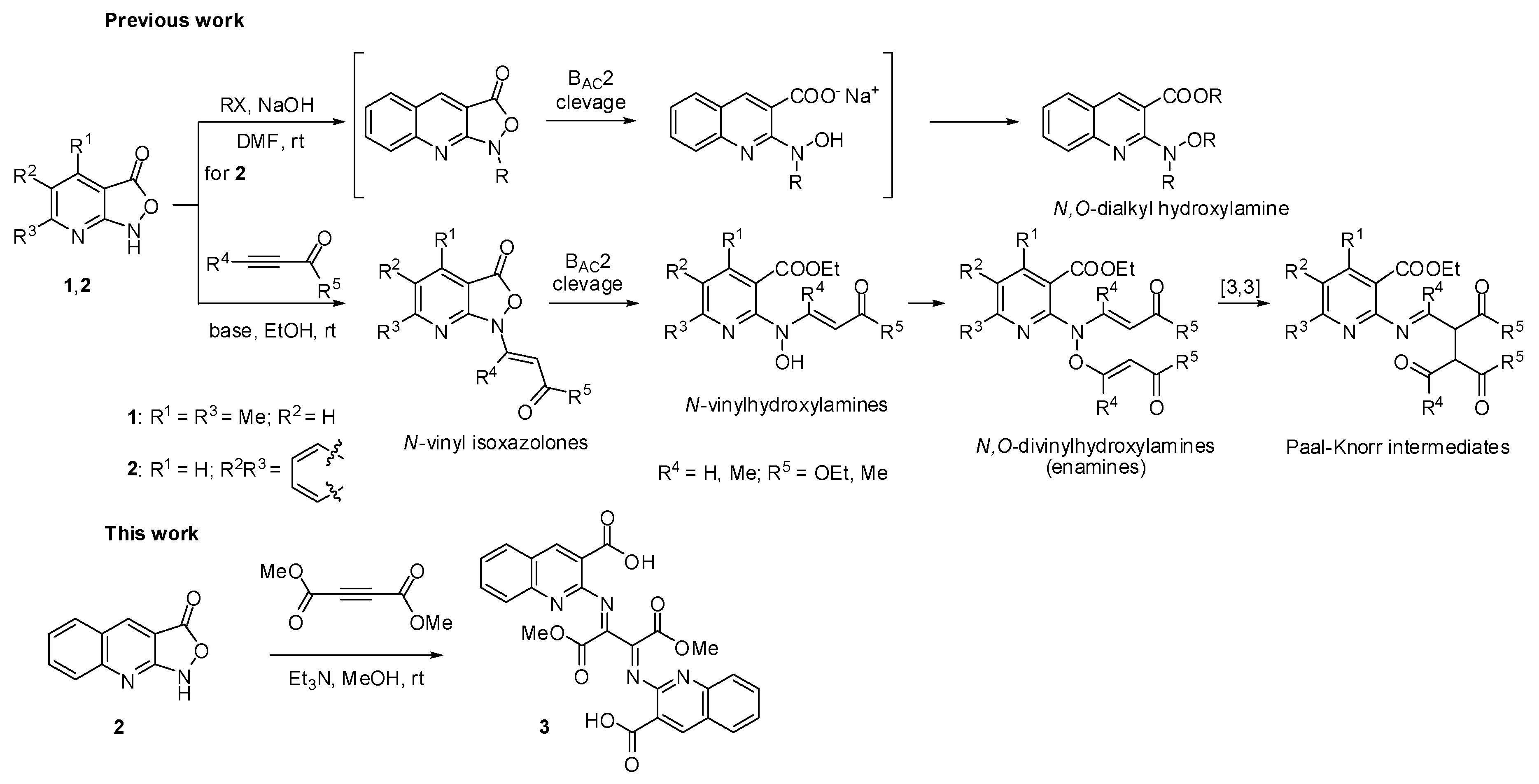
2. Results and discussion
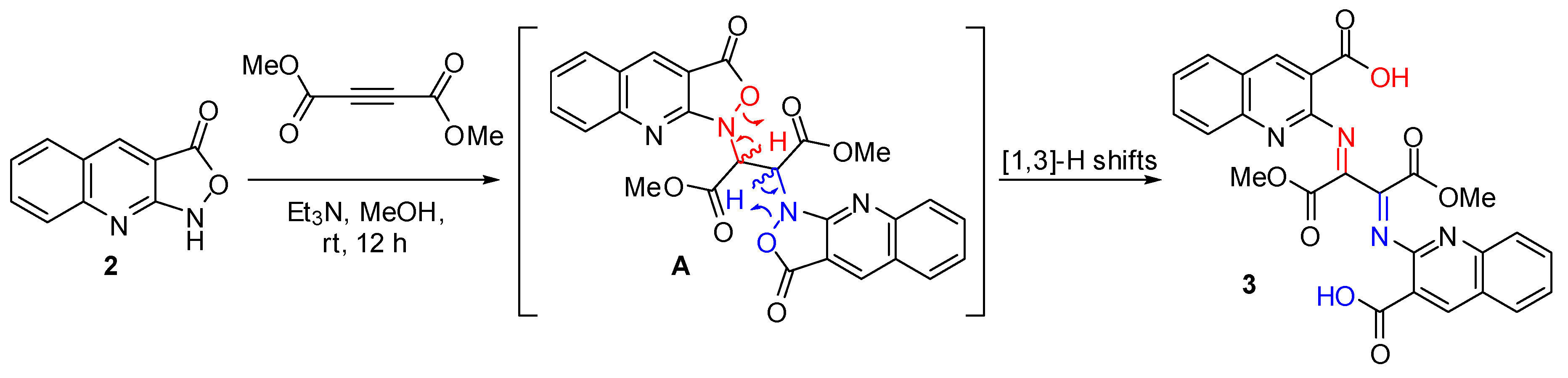
2.1. Chemistry
2.2. DFT Calculations
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of 2,2′-((1,4-dimethoxy-1,4-dioxobutane-2,3-diylidene)bis(azanylylidene))bis(quinoline-3-carboxylic acid) (3)
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ergenc, N.; Capan, G.; Otuk, G. New 4-arylhydrazono-5(4H)-isoxazolone derivatives as possible antibacterial and anticonvulsant agents. Pharmazie 1993, 48, 780–782. [Google Scholar]
- Mazimba, O.; Wale, K.; Loeto, D.; Kwape, T. Antioxidant and antimicrobial studies on fused-ring pyrazolones and isoxazolones. Bioorg. Med. Chem. 2014, 22, 6564–6569. [Google Scholar] [CrossRef]
- Chande, M.S.; Verma, R.S.; Barve, P.A.; Khanwelkar, R.R.; Vaidya, R.B.; Ajaikumar, K.B. Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: A Michael addition approach. Eur. J. Med. Chem. 2005, 40, 1143–1148. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, K.; Shi, X.; Wang, S.; Zhu, W.-M.; Zhang, X.-H. Purification and characterization of antibacterial compounds of Pseudoalteromonas flavipulchra JG1. Microbiology 2012, 158, 835–842. [Google Scholar] [CrossRef]
- Wierenga, W.; Evans, B.R.; Zurenko, G.E. Benzisoxazolones: Antimicrobial and antileukemic activity. J. Med. Chem. 1984, 27, 1212–1215. [Google Scholar] [CrossRef]
- Saidachary, G.; Veera Prasad, K.; Divya, D.; Singh, A.; Ramesh, U.; Sridhar, B.; China Raju, B. Convenient one-pot synthesis, anti-mycobacterial and anticancer activities of novel benzoxepinoisoxazolones and pyrazolones. Eur. J. Med. Chem. 2014, 76, 460–469. [Google Scholar] [CrossRef]
- Padmavathi, V.; Venkata Subbaiah, D.R.C.; Mahesh, K.; Radha Lakshmi, T. Synthesis and Bioassay of Amino-pyrazolone, Amino-isoxazolone and Amino-pyrimidinone Derivatives. Chem. Pharm. Bull. 2007, 55, 1704–1709. [Google Scholar] [CrossRef]
- Tanaka, K.; Matsuo, K.; Nakanishi, A.; Jo, M.; Shiota, H.; Yamaguchi, M.; Yoshino, S.; Kawaguchi, K. Syntheses and Antimicrobial Activities of Five-Membered Heterocycles Having a Phenylazo Substituent. Chem. Pharm. Bull. 1984, 32, 3291–3298. [Google Scholar] [CrossRef]
- Braunholtz, J.T.; Freeman, P.F.H. Fungicidal Isoxazolones. GB1074803, 5 July 1967. [Google Scholar]
- Das, N.P.; Mishra, P.K.; Sahu, S. Fungicidal activity of some substituted 5-isoxazolones. Acta Cienc. Indica Chem. 2011, 37, 239–243. [Google Scholar]
- Tabarki, M.A.; Besbes, R. Regioselective ring opening of β-phenylglycidate and aziridine-2-carboxylates with N-alkylhydroxylamines: Synthesis of isoxazolidinones. Tetrahedron 2014, 70, 1060–1064. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Eickhoff, H.; Hohn, E.; Bilitewski, U. Identification of inhibitors of yeast-to-hyphae transition in Candida albicans by a reporter screening assay. J. Biotechnol. 2013, 164, 137–142. [Google Scholar] [CrossRef]
- Miyake, T.; Yagasaki, Y.; Kagabu, S. Potential new fungicides: N-acyl-5-methyl-3(2H)-isoxazolone derivatives. J. Pest. Sci. 2012, 37, 89–94. [Google Scholar] [CrossRef]
- Kömürcü, S.G.; Rollas, S.; Yilmaz, N.; Cevikbas, A. Synthesis of 3-methyl-4-[(2,4-dihydro-4-substituted-3H-1,2,4-triazole-3-thione-5-yl)phenylhydrazono]-5-isoxazolone and evaluation of their antimicrobial activities. Drug Metabol. Drug Interact. 1995, 12, 161–169. [Google Scholar] [CrossRef]
- Summers, L.A. Agricultural fungicides. V. Preparation of 3-Methyl-4-(3′-pyridylhydrazono)-isoxazol-5-one and related compounds. Aust. J. Chem. 1973, 26, 2723–2725. [Google Scholar] [CrossRef]
- Cox, M.; Jahangri, S.; Perkins, M.V.; Prager, R.H. Some Synthetic Approaches to Glutamate AMPA Receptor Agonists Based on Isoxazolones. Aust. J. Chem. 2004, 57, 685–688. [Google Scholar] [CrossRef]
- Panathur, N.; Gokhale, N.; Dalimba, U.; Koushik, P.V.; Yogeeswari, P.; Sriram, D. New indole–isoxazolone derivatives: Synthesis, characterisation and in vitro SIRT1 inhibition studies. Bioorg. Med. Chem. Lett. 2015, 25, 2768–2772. [Google Scholar] [CrossRef]
- Rollas, S.; Kokyan, Ş.; Koçyiǧit-Kaymakçioǧlu, B.; Özbaş-Turan, S.; Akbuǧa, J. Synthesis and evaluation of cytotoxic activities of some substituted isoxazolone derivatives. Marmara Pharm. J. 2011, 15, 94–99. [Google Scholar] [CrossRef]
- Vergelli, C.; Schepetkin, I.A.; Crocetti, L.; Iacovone, A.; Giovannoni, M.P.; Guerrini, G.; Khlebnikov, A.I.; Ciattini, S.; Ciciani, G.; Quinn, M.T. Isoxazol-5(2H)-one: A new scaffold for potent human neutrophil elastase (HNE) inhibitors. J. Enzyme Inhib. Med. 2017, 32, 821–831. [Google Scholar] [CrossRef]
- Hou, X.Q.; Gao, Y.W.; Yang, S.T.; Wang, C.Y.; Ma, Z.Y.; Xia, X.Z. Role of macrophage migration inhibitory factor in influenza H5N1 virus pneumonia. Acta Virol. 2009, 53, 225–231. [Google Scholar] [CrossRef]
- Laughlin, S.K.; Clark, M.P.; Djung, J.F.; Golebiowski, A.; Brugel, T.A.; Sabat, M.; Bookland, R.G.; Laufersweiler, M.J.; VanRens, J.C.; Townes, J.A.; et al. The development of new isoxazolone based inhibitors of tumor necrosis factor-alpha (TNF-α) production. Bioorg. Med. Chem. Lett. 2005, 15, 2399–2403. [Google Scholar] [CrossRef]
- Deng, B.-L.; Hartman, T.L.; Buckheit, R.W., Jr.; Pannecouque, C.; De Clercq, E.; Cushman, M. Replacement of the Metabolically Labile Methyl Esters in the Alkenyldiarylmethane Series of Non-Nucleoside Reverse Transcriptase Inhibitors with Isoxazolone, Isoxazole, Oxazolone, or Cyano Substituents. J. Med. Chem. 2006, 49, 5316–5323. [Google Scholar] [CrossRef]
- Ishioka, T.; Tanatani, A.; Nagasawa, K.; Hashimoto, Y. Anti-Androgens with full antagonistic activity toward human prostate tumor LNCaP cells with mutated androgen receptor. Bioorg. Med. Chem. Lett. 2003, 13, 2655–2658. [Google Scholar] [CrossRef]
- Ishioka, T.; Kubo, A.; Koiso, Y.; Nagasawa, K.; Itai, A.; Hashimoto, Y. Novel Non-Steroidal/Non-Anilide Type Androgen Antagonists with an Isoxazolone Moiety. Bioorg. Med. Chem. 2002, 10, 1555–1566. [Google Scholar] [CrossRef]
- Laufer, S.A.; Margutti, S. Isoxazolone Based Inhibitors of p38 MAP Kinases. J. Med. Chem. 2008, 51, 2580–2584. [Google Scholar] [CrossRef]
- Demers, J.P.; Hageman, W.E.; Johnson, S.G.; Klaubert, D.H.; Look, R.A.; Moore, J.B. Selective inhibitors of protein kinase C in a model of graft-vs-host disease. Bioorg. Med. Chem. Lett 1994, 4, 2451–2456. [Google Scholar] [CrossRef]
- Kafle, B.; Aher, N.G.; Khadka, D.; Park, H.; Cho, H. Isoxazol-5(4H)one Derivatives as PTP1B Inhibitors Showing an Anti-Obesity Effect. Chem. Asian J. 2011, 6, 2073–2079. [Google Scholar] [CrossRef]
- Kafle, B.; Cho, H. Isoxazolone Derivatives as Potent Inhibitors of PTP1B. Bull. Korean Chem. Soc. 2012, 33, 275–277. [Google Scholar] [CrossRef]
- Frolund, B.; Kristiansen, U.; Brehm, L.; Hansen, A.B.; Krogsgaard-Larsen, P.; Falch Partial, E. Partial GABAA Receptor Agonists. Synthesis and in Vitro Pharmacology of a Series of Nonannulated Analogs of 4,5,6,7-Tetrahydroisoxazolo[4,5-c]pyridin-3-ol. J. Med. Chem. 1995, 38, 3287–3296. [Google Scholar] [CrossRef]
- Tamura, N.; Matsushita, Y.; Kishimoto, S.; Itoh, K. Synthesis and Biological Activity of (S)-2-Amino-3-(2, 5-dihydro-5-oxo-4-isoxazolyl)propanoic Acid (TAN-950 A) Derivatives. Chem. Pharm. Bull. 1991, 39, 1199–1212. [Google Scholar] [CrossRef][Green Version]
- Tamura, N.; Itoh, K.; Iwama, T. Synthesis and Glutamate-Agonistic Acitivity of (S)-2-Amino-3-(2, 5-dihydro-5-oxo-3-isoxazolyl)-propanoic Acid Derivatives. Chem. Pharm. Bull. 1992, 40, 381–386. [Google Scholar] [CrossRef]
- Toshi, I.; Yasuo, N.; Norikazu, T.; Setsuo, H.; Akinobu, N. A novel glutamate agonist, TAN-950 A, isolated from streptomycetes. Eur. J. Pharmacol. 1991, 197, 187–192. [Google Scholar] [CrossRef]
- Shinabarger, D. Mechanism of action of the oxazolidinone antibacterial agents. Exp. Opin. Investig. Drugs 1999, 8, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Sączewski, J.; Jalińska, A.; Kędzia, A. New derivatives of 4,6-dimethylisoxazolo[3,4-b] pyridin-3(1H)-one: Synthesis, tautomerism, electronic structure and antibacterial activity. Heterocycl. Commun 2014, 20, 215–223. [Google Scholar] [CrossRef]
- Sączewski, J.; Fedorowicz, J.; Kędzia, A.; Ziółkowska-Klinkosz, M.; Jalińska, A. Synthesis and Antifungal Activity of Some 4,6-Dimethylisoxazolo[3,4-b]pyridin-3(1H)-one Derivatives. Med. Chem. 2016, 12, 640–646. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomašič, T.; Skok, Ž.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef]
- Ciura, K.; Fedorowicz, J.; Andrić, F.; Greber, K.E.; Gurgielewicz, A.; Sawicki, W.; Sączewski, J. Lipophilicity Determination of Quaternary (Fluoro)Quinolones by Chromatographic and Theoretical Approaches. Int. J. Mol. Sci. 2019, 20, 5288. [Google Scholar] [CrossRef]
- Sączewski, J.; Fedorowicz, J.; Korcz, M.; Sączewski, F.; Wicher, B.; Gdaniec, M.; Konopacka, A. Experimental and theoretical studies on the tautomerism and reactivity of isoxazolo[3,4-b]quinolin-3(1H)-ones. Tetrahedron 2015, 71, 8975–8984. [Google Scholar] [CrossRef]
- Sączewski, J.; Fedorowicz, J.; Gdaniec, M.; Wiśniewska, P.; Sieniawska, E.; Drażba, Z.; Rzewnicka, J.; Balewski, Ł. The Elusive Paal–Knorr Intermediates in the Trofimov Synthesis of Pyrroles: Experimental and Theoretical Studies. J. Org. Chem. 2017, 82, 9737–9743. [Google Scholar] [CrossRef]
- Sączewski, J.; Hinc, K.; Obuchowski, M.; Gdaniec, M. The Tandem Mannich–Electrophilic Amination Reaction: A Versatile Platform for Fluorescent Probing and Labeling. Chem. Eur. J. 2013, 19, 11531–11535. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Sączewski, J.; Drażba, Z.; Wiśniewska, P.; Gdaniec, M.; Wicher, B.; Suwiński, G.; Jalińska, A. Synthesis and fluorescence of dihydro-[1,2,4]triazolo[4,3-a]pyridin-2-iumcarboxylates: An experimental and TD-DFT comparative study. Dyes Pigments 2019, 161, 347–359. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A density functional with spherical atom dispersion terms. J. Chem. Theor. Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
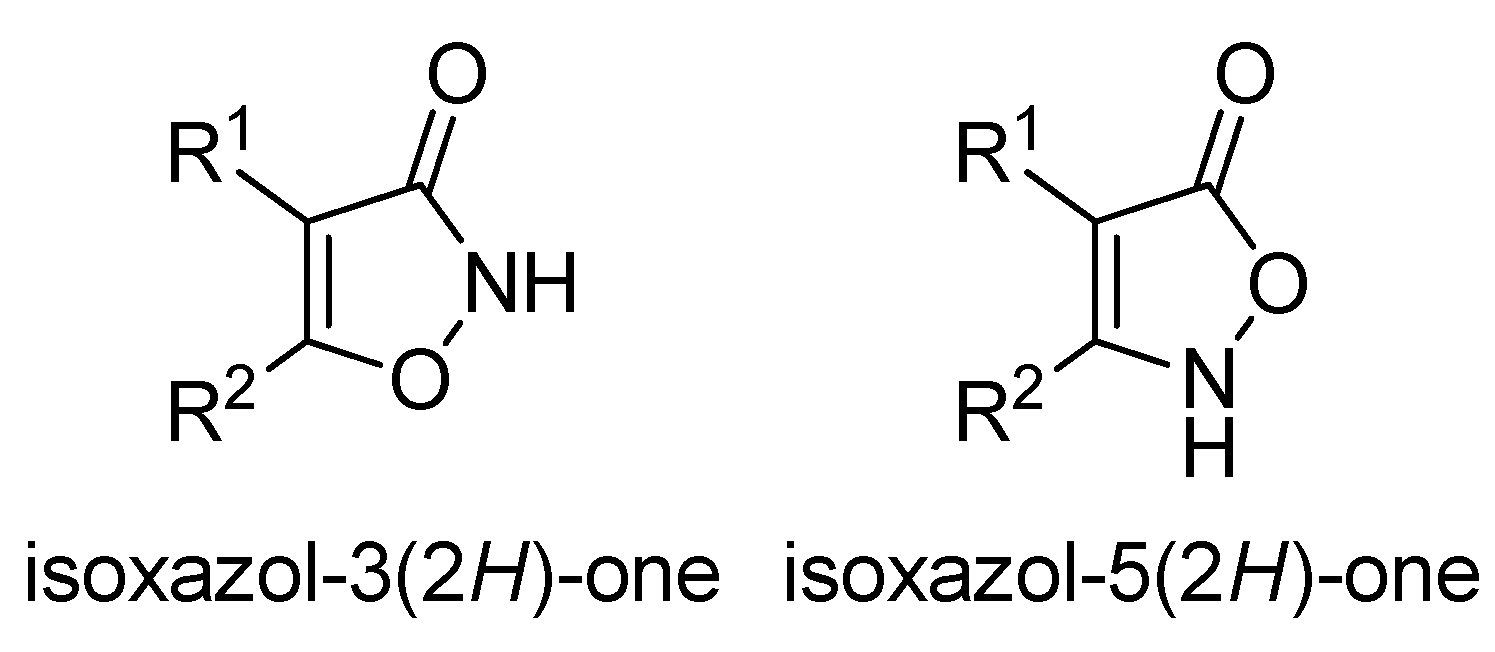
| Relative Energy |  | 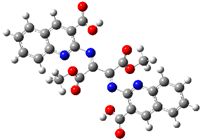 |
|---|---|---|
| Vacuum | A | 3 |
| ΔE (kcal/mol) | ||
| B3LYP | 0 | −46.5 |
| ωB97XD | 0 | −40.5 |
| APFD | 0 | −36.8 |
| ΔG (kcal/mol) | ||
| B3LYP | 0 | −50.9 |
| ωB97XD | 0 | −45.7 |
| APFD | 0 | −40.6 |
| MeOH | A | 3 |
| ΔE (kcal/mol) | ||
| B3LYP | 0 | −47.8 |
| ωB97XD | 0 | −42.2 |
| APFD | 0 | −38.4 |
| ΔG (kcal/mol) | ||
| B3LYP | 0 | −51.4 |
| ωB97XD | 0 | −47.4 |
| APFD | 0 | −41.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorowicz, J.; Gzella, K.; Wiśniewska, P.; Sączewski, J. 2,2′-((1,4-Dimethoxy-1,4-dioxobutane-2,3-diylidene)bis(azanylylidene))bis(quinoline-3-carboxylic acid). Molbank 2019, 2019, M1093. https://doi.org/10.3390/M1093
Fedorowicz J, Gzella K, Wiśniewska P, Sączewski J. 2,2′-((1,4-Dimethoxy-1,4-dioxobutane-2,3-diylidene)bis(azanylylidene))bis(quinoline-3-carboxylic acid). Molbank. 2019; 2019(4):M1093. https://doi.org/10.3390/M1093
Chicago/Turabian StyleFedorowicz, Joanna, Karol Gzella, Paulina Wiśniewska, and Jarosław Sączewski. 2019. "2,2′-((1,4-Dimethoxy-1,4-dioxobutane-2,3-diylidene)bis(azanylylidene))bis(quinoline-3-carboxylic acid)" Molbank 2019, no. 4: M1093. https://doi.org/10.3390/M1093
APA StyleFedorowicz, J., Gzella, K., Wiśniewska, P., & Sączewski, J. (2019). 2,2′-((1,4-Dimethoxy-1,4-dioxobutane-2,3-diylidene)bis(azanylylidene))bis(quinoline-3-carboxylic acid). Molbank, 2019(4), M1093. https://doi.org/10.3390/M1093