N-Benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide
Abstract
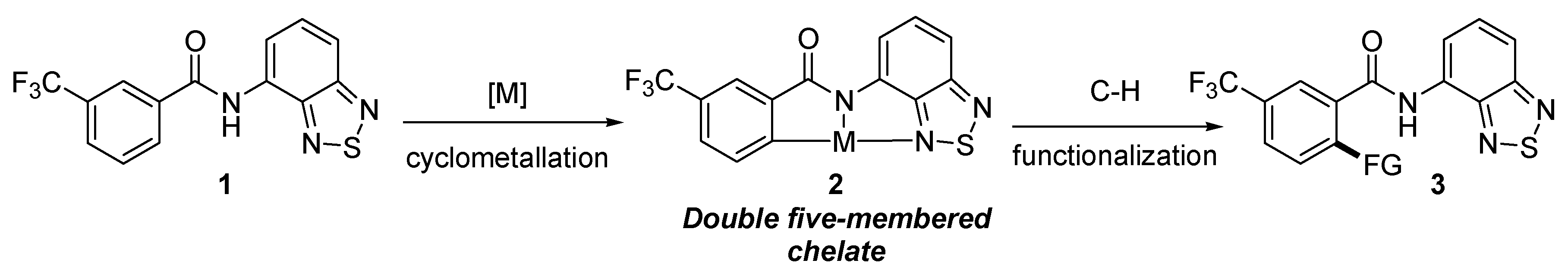
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
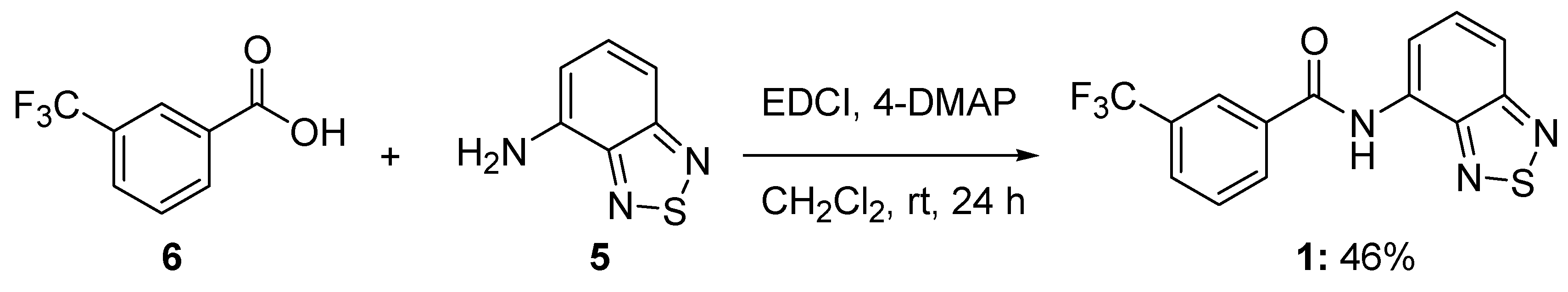
3.2. Synthesis of N-benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide (1)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shilov, A.E.; Shul’pin, G.B. Activation of C-H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.R.; Sanford, M.S. Transition Metal Catalyzed Oxidative Functionalization of Carbon-Hydrogen Bonds. Tetrahdedron 2006, 62, 2439–2463. [Google Scholar] [CrossRef]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef] [PubMed]
- Rouquet, G.; Chatani, N. Catalytic Functionalization of C(sp2)–H and C(sp3)–H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Intramolecular Five-Membered Ring Compounds and Their Applications. Coord. Chem. Rev. 2004, 248, 995–1023. [Google Scholar] [CrossRef]
- Reddy, C.; Bisht, N.; Parella, R.; Babu, S.A. 4-Amino-2,1,3-benzothiadiazole as a Removable Bidentate Directing Group for the Pd(II)-Catalyzed Arylation/Oxygenation of sp2/sp3 β-C–H Bonds of Carboxamides. J. Org. Chem. 2016, 81, 12143–12168. [Google Scholar] [CrossRef] [PubMed]
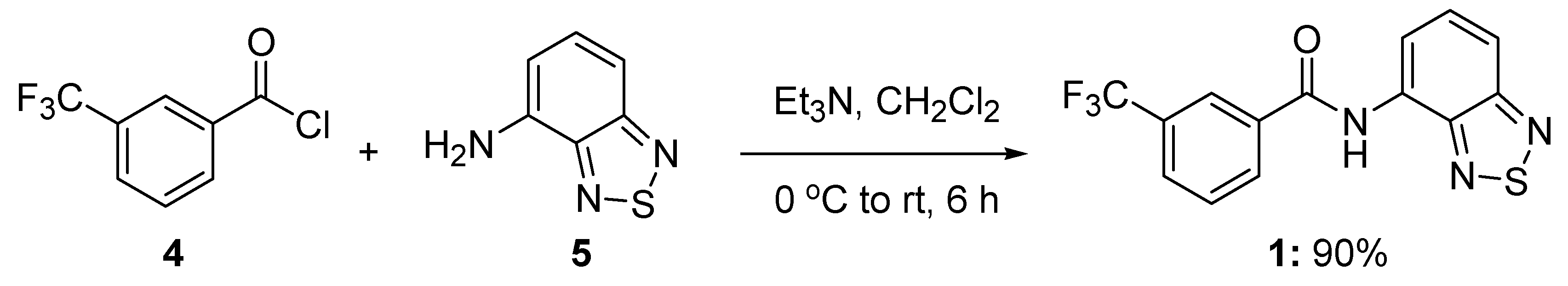
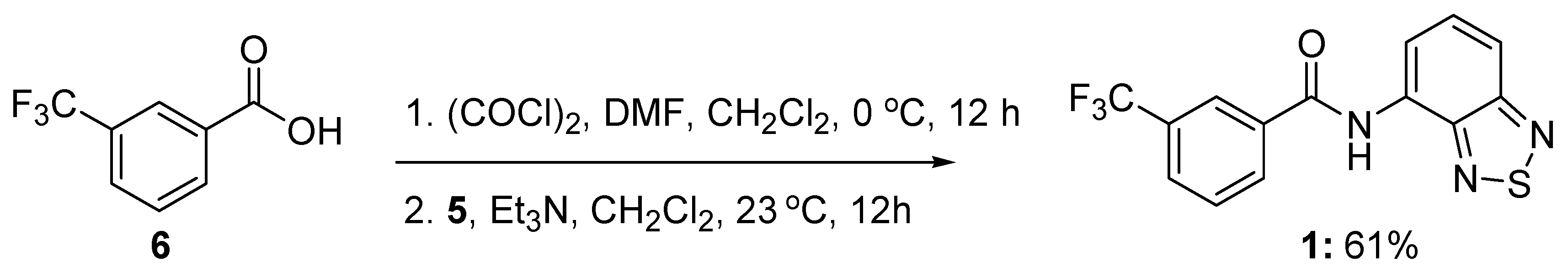
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mamari, H.H.; Al Awaimri, N.; Al Lawati, Y. N-Benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide. Molbank 2019, 2019, M1075. https://doi.org/10.3390/M1075
Al Mamari HH, Al Awaimri N, Al Lawati Y. N-Benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide. Molbank. 2019; 2019(3):M1075. https://doi.org/10.3390/M1075
Chicago/Turabian StyleAl Mamari, Hamad H., Nasser Al Awaimri, and Yousuf Al Lawati. 2019. "N-Benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide" Molbank 2019, no. 3: M1075. https://doi.org/10.3390/M1075
APA StyleAl Mamari, H. H., Al Awaimri, N., & Al Lawati, Y. (2019). N-Benzo[c][1,2,5]thiazol-4-yl-3-trifluoromethylbenzamide. Molbank, 2019(3), M1075. https://doi.org/10.3390/M1075




