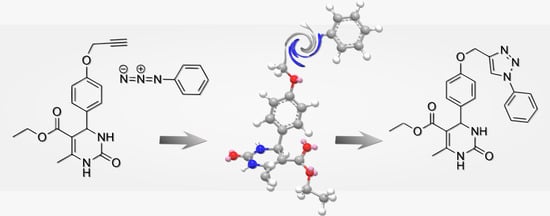
Ethyl 6-Methyl-2-oxo-4-{4-[(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy]phenyl}-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
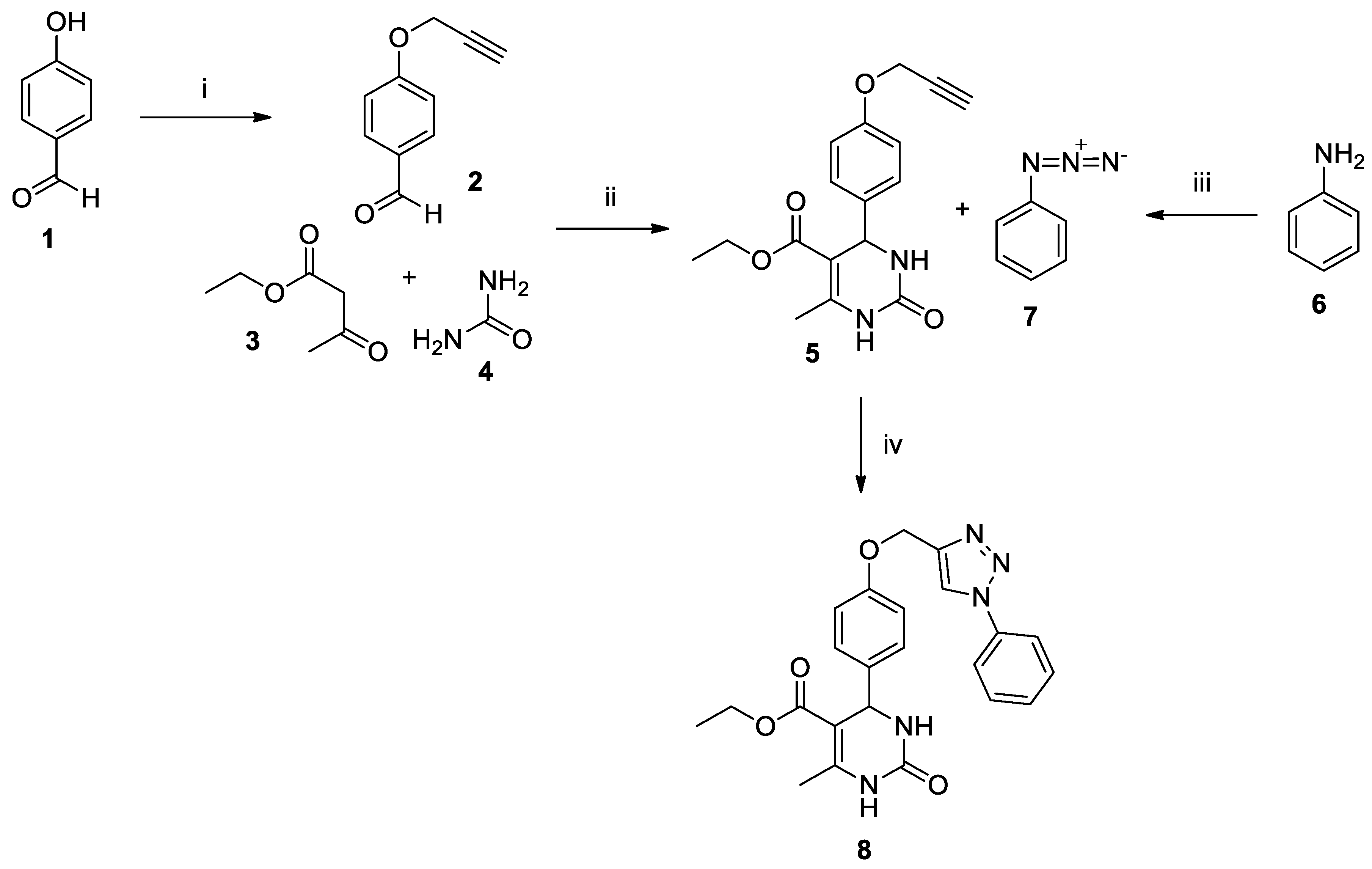
3.2. Synthesis of Aromatic Azide (7)
3.3. Synthesis of 4-(Prop-2-yn-1-yloxy)benzaldehyde (2)
3.4. Synthesis of 3,4-Dihydropyrimidinone (5)
3.5. Synthesis of Hybrid 3,4-Dihydropyrimidinone-triazole LaSOM® 293 (8)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Gonçalves, I.L.; Davi, L.; Rockenbach, L.; das Neves, G.M.; Kagami, L.P.; Canto, R.F.S.; Figueiró, F.; Battastini, A.M.O.; Eifler-Lima, V.L. Versatility of the Biginelli reaction: Synthesis of new biphenyl dihydropyrimidin-2-thiones using different ketones as building blocks. Tetrahedron Lett. 2018, 59, 2759–2762. [Google Scholar] [CrossRef]
- Matos, L.H.S.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological activity of dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; Rockenbach, L.; das Neves, G.M.; Göethel, G.; Nascimento, F.; Porto Kagami, L.; Figueiró, F.; Oliveira de Azambuja, G.; de Fraga Dias, A.; Amaro, A.; et al. Effect of N-1 arylation of monastrol on kinesin Eg5 inhibition in glioma cell lines. MedChemComm 2018, 9, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Wang, Y. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Tamimi, M.; Yahyavi, H.; Hosseinnejad, T. Huisgen’s cycloaddition reactions: A full perspective. Curr. Org. Chem. 2016, 20, 1591–1647. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- González-Olvera, R.; Román-Rodríguez, V.; Negrón-Silva, G.E.; Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J.; Santillan, R. Multicomponent Synthesis and Evaluation of New 1,2,3-Triazole Derivatives of Dihydropyrimidinones as Acidic Corrosion Inhibitors for Steel. Molecules 2016, 21, 250. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Dabiri, M.; Koohshari, M.; Movahed, S.K.; Bararjanian, M. One-pot synthesis of 1,2,3-triazole linked dihydropyrimidinones via Huisgen 1,3-dipolar/Biginelli cycloaddition. Mol. Divers. 2011, 15, 833–837. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, I.L.; Azambuja, G.O.d.; Davi, L.; Gonçalves, G.A.; Kagami, L.P.; Neves, G.M.d.; Silveira, J.P.; Canto, R.F.S.; Eifler-Lima, V.L. Ethyl 6-Methyl-2-oxo-4-{4-[(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy]phenyl}-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2019, 2019, M1076. https://doi.org/10.3390/M1076
Gonçalves IL, Azambuja GOd, Davi L, Gonçalves GA, Kagami LP, Neves GMd, Silveira JP, Canto RFS, Eifler-Lima VL. Ethyl 6-Methyl-2-oxo-4-{4-[(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy]phenyl}-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank. 2019; 2019(3):M1076. https://doi.org/10.3390/M1076
Chicago/Turabian StyleGonçalves, Itamar Luís, Gabriel Oliveira de Azambuja, Leonardo Davi, Guilherme Arraché Gonçalves, Luciano Porto Kagami, Gustavo Machado das Neves, João Pedro Silveira, Rômulo Faria Santos Canto, and Vera Lucia Eifler-Lima. 2019. "Ethyl 6-Methyl-2-oxo-4-{4-[(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy]phenyl}-1,2,3,4-tetrahydropyrimidine-5-carboxylate" Molbank 2019, no. 3: M1076. https://doi.org/10.3390/M1076
APA StyleGonçalves, I. L., Azambuja, G. O. d., Davi, L., Gonçalves, G. A., Kagami, L. P., Neves, G. M. d., Silveira, J. P., Canto, R. F. S., & Eifler-Lima, V. L. (2019). Ethyl 6-Methyl-2-oxo-4-{4-[(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy]phenyl}-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank, 2019(3), M1076. https://doi.org/10.3390/M1076