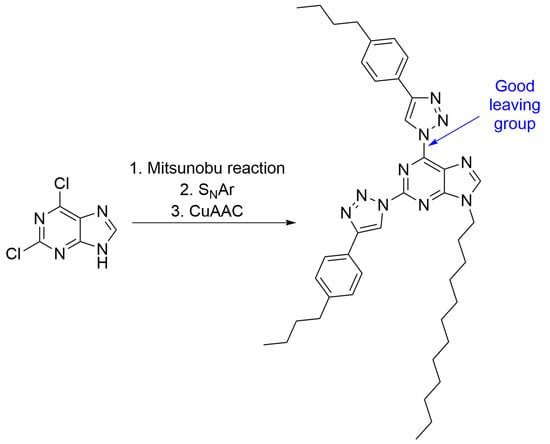
2,6-Bis[4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl]-9-dodecyl-9H-purine
Abstract
1. Introduction
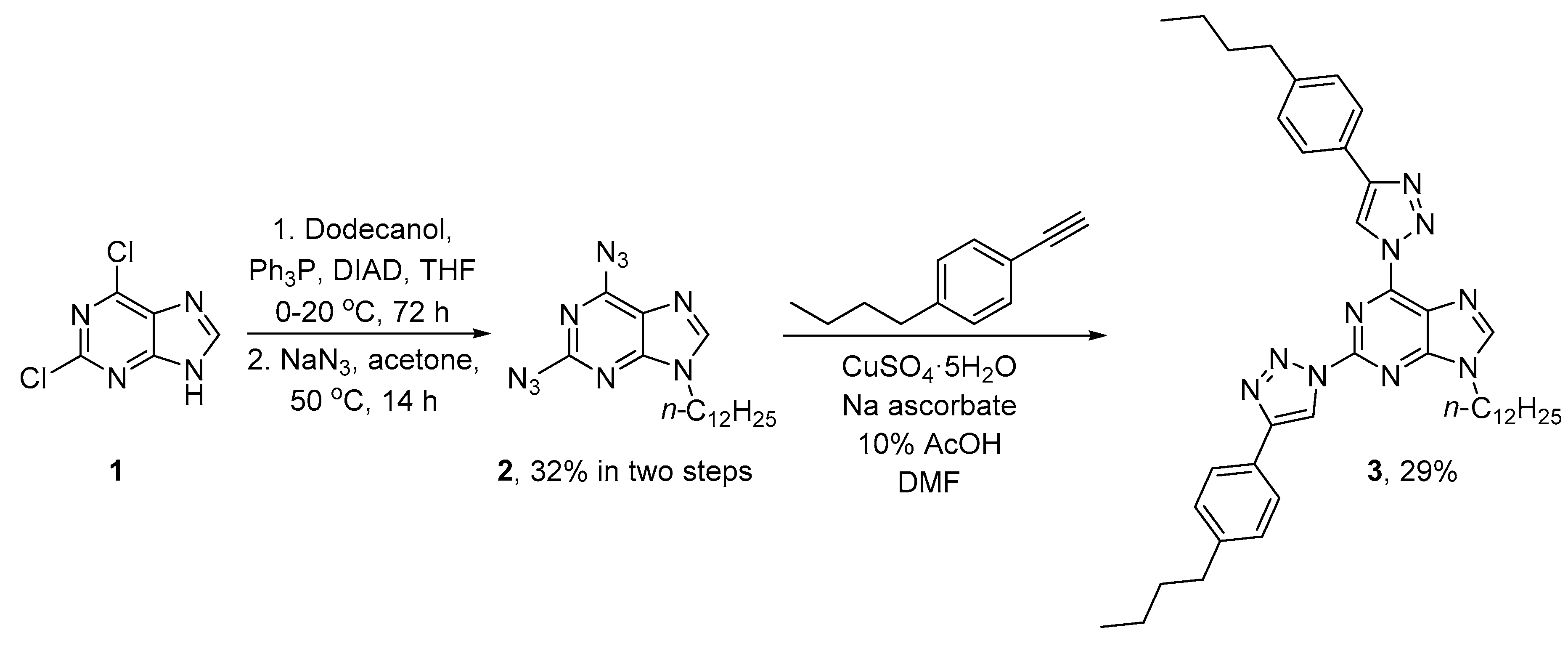
2. Results and Discussion
3. Materials and Methods
3.1. General Information
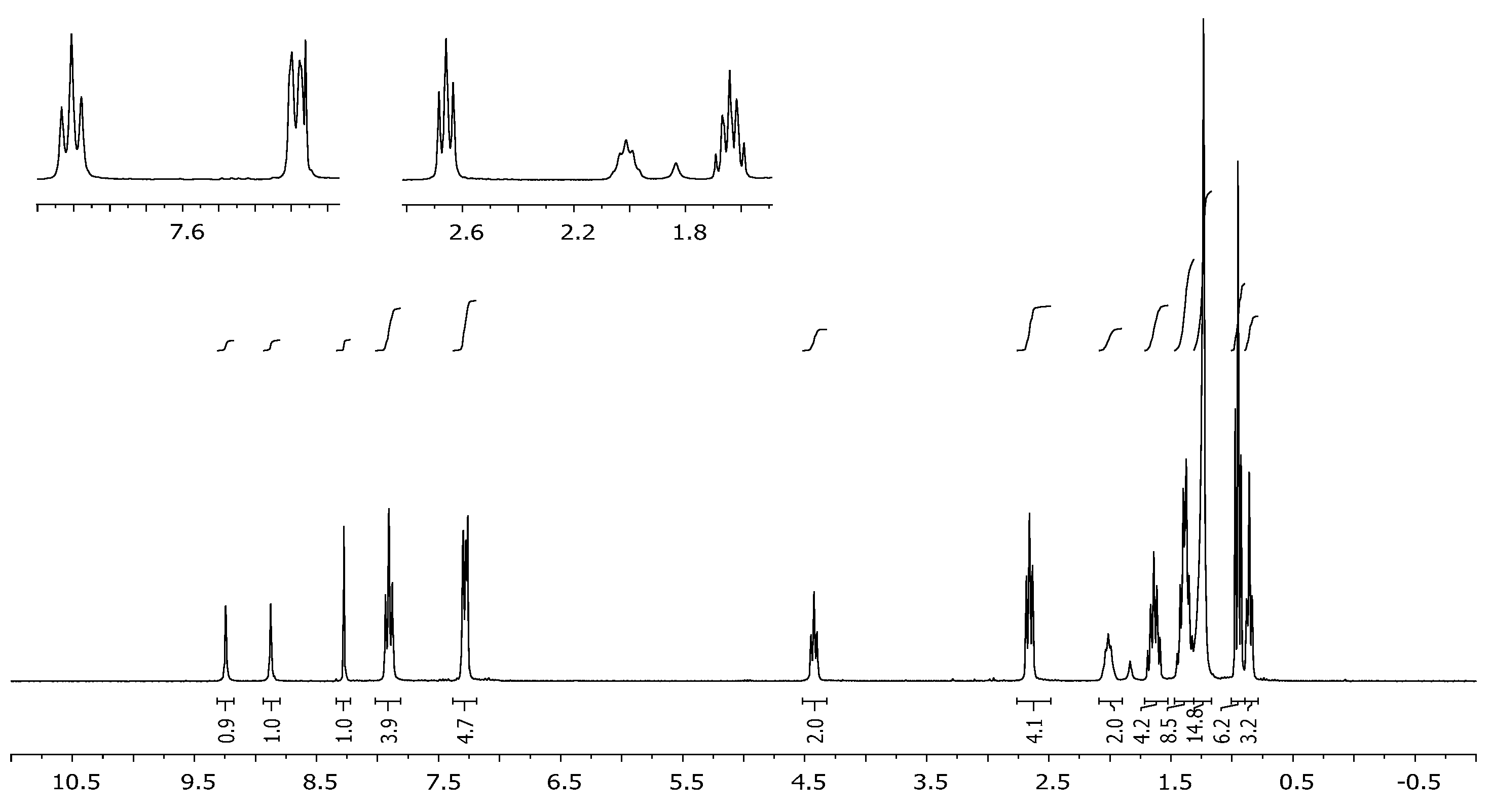
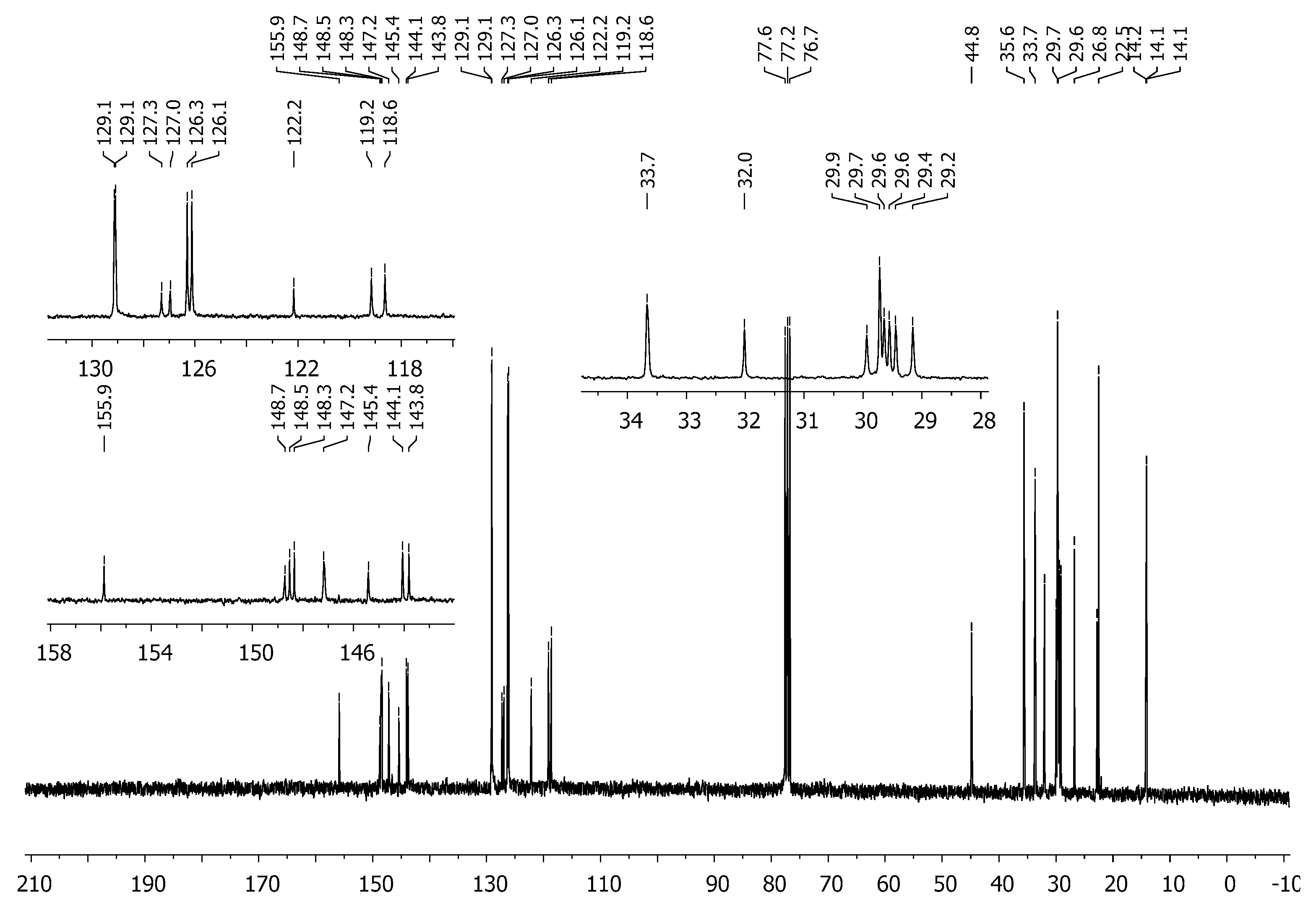
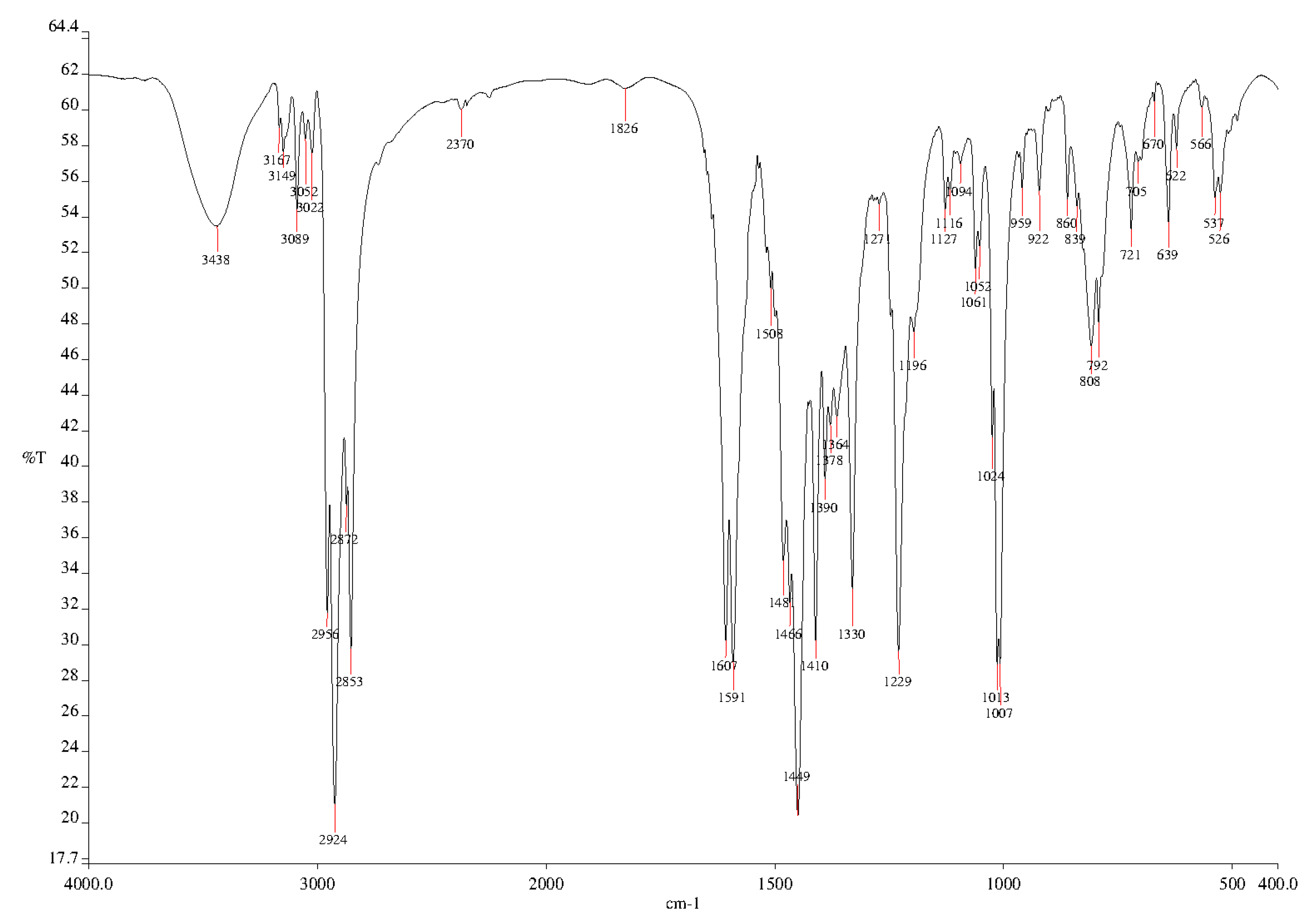
3.2. Synthesis of 2,6-Bis[4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl]-9-dodecyl-9H-purine (3)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robak, P.; Robak, T. Older and New Purine Nucleoside Analogs for Patients with Acute Leukemias. Cancer Treat. Rev. 2013, 39, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Latocha, M.; Pluta, K. Synthesis and Anticancer Activity of Thiosubstituted Purines. Med. Chem. Res. 2015, 24, 3107–3116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shelton, J.; Lu, X.; Hollenbaugh, J.A.; Cho, J.H.; Amblard, F.; Schinazi, R.F. Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs. Chem. Rev. 2016, 116, 14379–14455. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Carter, K.; Janeba, Z.; Sampath, A.; Schang, L.M.; Tarbet, E.B.; Vere Hodge, R.A.; Bray, M.; Esté, J.A. Highlights of the 30th International Conference on Antiviral Research. Antivir. Res. 2017, 145, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Manvar, A.; Shah, A. Diversity Oriented Efficient Access of Trisubstituted Purines via Sequential Regioselective Mitsunobu Coupling and SNAr Based C6 Functionalizations. Tetrahedron 2013, 69, 680–691. [Google Scholar] [CrossRef]
- De Clercq, E. Recent Highlights in the Development of New Antiviral Drugs. Curr. Opin. Microbiol. 2005, 8, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Linden, J.; Mu, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmocol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Minetti, P.; Tinti, M.O.; Carminati, P.; Castorina, M.; Assunta, M.; Cesare, D.; Di Serio, S.; Gallo, G.; Ghirardi, O.; Giorgi, F.; et al. 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and Analogues as A2A Adenosine Receptor Antagonists. Design, Synthesis, and Pharmacological Characterization. J. Med. Chem. 2005, 48, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Kovaļovs, A.; Novosjolova, I.; Bizdēna, Ē.; Bižāne, I.; Skardziute, L.; Kazlauskas, K.; Jursenas, S.; Turks, M. 1,2,3-Triazoles as Leaving Groups in Purine Chemistry: A Three-Step Synthesis of N6-Substituted-2-triazolyl-adenine Nucleosides and Photophysical Properties Thereof. Tetrahedron Lett. 2013, 54, 850–853. [Google Scholar] [CrossRef]
- Novosjolova, I.; Bizdēna, Ē.; Turks, M. Application of 2,6-Diazidopurine Derivatives in the Synthesis of Thiopurine Nucleosides. Tetrahedron Lett. 2013, 54, 6557–6561. [Google Scholar] [CrossRef]
- Šišuļins, A.; Bucevičius, J.; Tseng, Y.-T.; Novosjolova, I.; Traskovskis, K.; Bizdēna, Ē.; Chang, H.-T.; Tumkevičius, S.; Turks, M. Synthesis and Fluorescent Properties of N(9)-Alkylated 2-Amino-6-triazolylpurines and 7-Deazapurines. Beilstein J. Org. Chem. 2019, 15, 474–489. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šišuļins, A.; Bizdēna, Ē.; Turks, M.; Novosjolova, I. 2,6-Bis[4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl]-9-dodecyl-9H-purine. Molbank 2019, 2019, M1073. https://doi.org/10.3390/M1073
Šišuļins A, Bizdēna Ē, Turks M, Novosjolova I. 2,6-Bis[4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl]-9-dodecyl-9H-purine. Molbank. 2019; 2019(3):M1073. https://doi.org/10.3390/M1073
Chicago/Turabian StyleŠišuļins, Andrejs, Ērika Bizdēna, Māris Turks, and Irina Novosjolova. 2019. "2,6-Bis[4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl]-9-dodecyl-9H-purine" Molbank 2019, no. 3: M1073. https://doi.org/10.3390/M1073
APA StyleŠišuļins, A., Bizdēna, Ē., Turks, M., & Novosjolova, I. (2019). 2,6-Bis[4-(4-butylphenyl)-1H-1,2,3-triazol-1-yl]-9-dodecyl-9H-purine. Molbank, 2019(3), M1073. https://doi.org/10.3390/M1073