Synthesis of 1H-3-{4-[(3-Dimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole and Evaluation of Its Antiprotozoal Activity
Abstract
1. Introduction
2. Results and Discussion
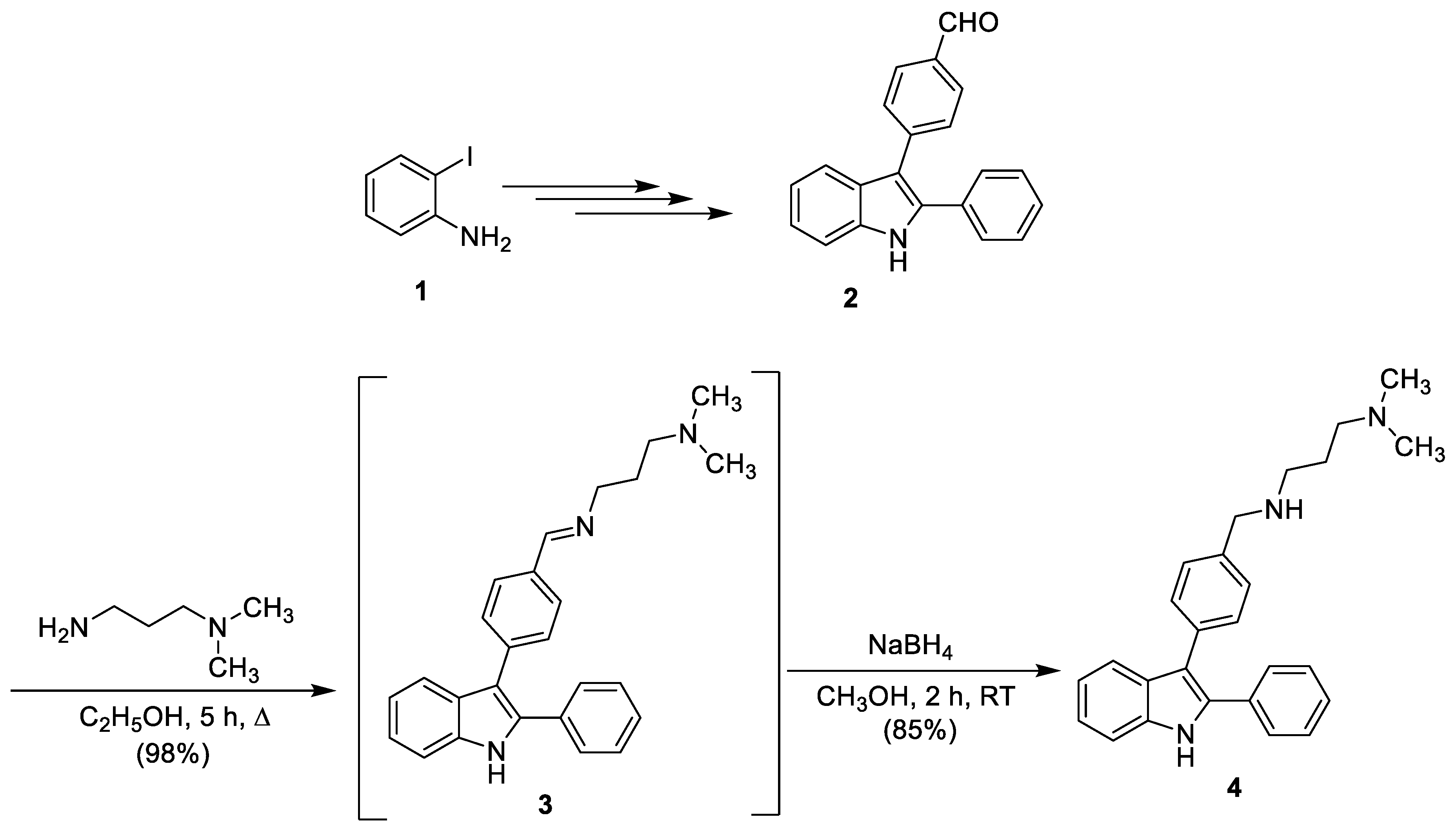
2.1. 1H-3-{4-[(3-Ddimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole
2.2. Antiprotozoal Activity
3. Materials and Methods
3.1. 1H-3-{4-[(3-Dimethylaminopropyl)iminomethyl]phenyl}-2-phenylindole (3)
3.2. 1H-3-{4-[(3-Dimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole (4)
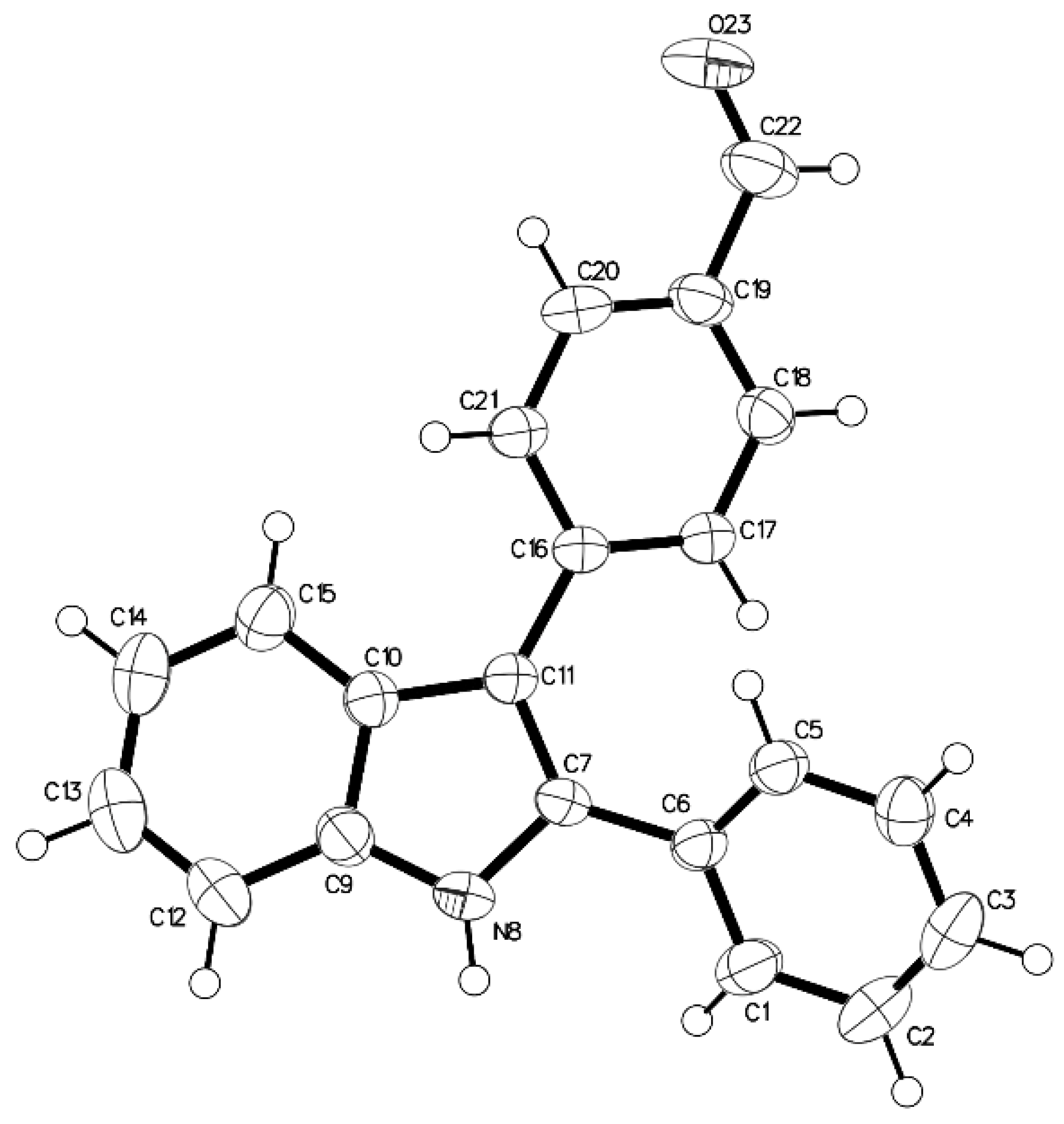
3.3. X-ray Data
3.4. In Vitro Antiplasmodial Activity
3.5. In Vitro Antileishmanial Activity
3.6. In Vitro Antitrypanosomal Activity
3.7. Cytotoxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Norwood, V.M.; Huigens, R.W. Harnessing the chemistry of the indole heterocycle to drive discoveries in biology and medicine. ChemBioChem 2019. [Google Scholar] [CrossRef] [PubMed]
- Shafakat Alia, N.A.; Darab, B.A.; Pradhana, V.; Farooquia, M. Chemistry and Biology of indoles and Indazoles: A mini-review. Mini-Rev. Med. Chem. 2013, 13, 1792–1800. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Ha Choi, E. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Biswal, S.; Sahoo, U.; Sethy, S.; Kumar, H.K.S.; Banerjee, M. Indole: The molecule of diverse biological activities. Asian J. Pharm. Clin. Res. 2012, 5, 1–6. [Google Scholar]
- Johansson, H.; Bøgeløv Jørgensen, T.; Gloriam, D.E.; Braüner-Osborne, H.; Sejer Pedersen, D. 3-Substituted 2-phenyl-indoles: privileged structures for medicinal chemistry. RSC Adv. 2013, 3, 945–960. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, Q.; Yang, G.F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef]
- Kim, J.; Park, W. Indole: A signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J. Microbiol. 2015, 53, 421–428. [Google Scholar] [CrossRef]
- Melander, R.J.; Minvielle, M.J.; Melander, C. Controlling bacterial behavior with indole-containing natural products and derivatives. Tetrahedron 2014, 70, 6363–6372. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Kaur, A.; Goyal, B. Benzofuran and Indole: Promising Scaffolds for Drug Development in Alzheimer’s Disease. ChemMedChem 2018, 13, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sakr, W.A.; Rahman, K.M. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets 2010, 11, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, P.N.; Karad, S.C.; Raval, D.K. A review on diverse heterocyclic compounds as the privileged scaffolds in antimalarial drug discovery. Eur. J. Med. Chem. 2018, 158, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Design of antimalarial agents based on natural products. Curr. Org. Chem. 2017, 21, 1824–1846. [Google Scholar] [CrossRef]
- Xu, Y.J.; Pieters, L. Recent developments in antimalarial natural products isolated from medicinal plants. Mini-Rev. Med. Chem. 2013, 13, 1056–1072. [Google Scholar] [CrossRef]
- Barnett, D.S.; Guy, R.K. Antimalarials in Development in 2014. Chem. Rev. 2014, 114, 11221–11241. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, antimalarial activity and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzym. Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef]
- Guillon, J.; Cohen, A.; Nath Das, R.; Boudot, C.; Meriem Gueddouda, N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Tisnerat, C.; et al. Design, synthesis, and antiprotozoal evaluation of new 2,9-bis[(substituted-aminomethyl)phenyl]-1,10-phenanthroline derivatives. Chem. Biol. Drug Des. 2018, 91, 974–995. [Google Scholar] [CrossRef]
- Arcadia, A.; Cacchi, S.; Marinelli, F. A versatile approach to 2,3-disubstituted indoles through the palladium-catalysed cyclization of o-alkynyltrifluoroacetanilides with vinyl triflates and aryl halides. Tetrahedron Lett. 1992, 33, 3915–3918. [Google Scholar]
- Cacchi, S.; Fabrizi, G.; Lamba, D.; Marinelli, F.; Parisi, L.M. 2-Subsitutedd 3-aryl- and 3-heteroarylindoles by the palladium-catalyzed reaction of o-trifluoroacetanilides with aryl bromides and triflates? Synthesis 2003, 728–734. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Parisi, L.M. Preparation of indoles from o-alkynyltrifluoroacetanilides through the aminopalladium-reductive elimination process. Synthesis 2004, 2004, 1889–1894. [Google Scholar] [CrossRef]
- Supplementary X-ray crystallographic data: Cambridge Crystallographic Data Centre, University Chemical Lab, Lensfield Road, Cambridge, CB2 1EW, UK; E-mail: deposit@chemcrys.cam.ac.uk.
- Sheldrick, G.M. (1996) SADABS, University of Göttingen, Germany.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef]
- Bennett, T.N.; Paguio, M.; Gligorijevic, B.; Seudieu, C.; Kosar, A.D.; Davidson, E.; Roepe, P.D. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob. Agents Chemother. 2004, 48, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Bacon, D.J.; Latour, C.; Lucas, C.; Colina, O.; Ringwald, P.; Picot, S. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob. Agents Chemother. 2007, 51, 1172–1178. [Google Scholar] [CrossRef]
- Kaddouri, H.; Nakache, S.; Houzé, S.; Mentré, F.; Le Bras, J. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob. Agents Chemother. 2006, 50, 3343–3349. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Emami, S.A.; Zamanai Taghizadeh Rabe, S.; Ahi, A.; Mahmoudi, M. Inhibitory Activity of Eleven Artemisia Species from Iran against Leishmania Major Parasites. Iran J. Basic Med. Sci. 2012, 15, 807–811. [Google Scholar]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Baltz, T.; Baltz, D.; Giroud, C. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
| Compound | P. falciparum strains IC50 Values (μM) a | L. donovani IC50 Values (μM) b | Trypanosoma brucei brucei Strain Antat 1.9 IC50 Values (μM) c | Cytotoxicity to HepG2 Cells CC50 Values (μM) d | |
|---|---|---|---|---|---|
| W2 | 3D7 | ||||
| Chloroquine e | 0.40 ± 0.04 | 0.11 ± 0.01 | n.d. h | n.d. h | 30 |
| Mefloquine e | 0.016 ± 0.002 | 0.06 ± 0.003 | n.d. h | n.d. h | n.d. h |
| Pentamidine f | n.d. h | n.d. h | 5.5 ± 0.8 | 0.0002 ± 0.00006 | 2.3 ± 0.5 |
| Amphotericin B f | n.d. h | n.d. h | 0.25 ± 0.01 | n.d. h | 8.8 ± 0.6 |
| Suramine g | n.d. h | n.d. h | n.d. h | 0.03 ± 0.003 | n.d. h |
| Fexinidazole g | n.d. h | n.d. h | n.d. h | 0.59 ± 0.039 | n.d. h |
| Eflornithine g | n.d. h | n.d. h | n.d. h | 15.19 ± 0.64 | n.d. h |
| Doxorubicin | n.d. h | n.d. h | n.d. h | n.d. h | 0.17 ± 0.03 |
| 4 | 1.80 ± 0.10 | 1.10 ± 0.12 | 3.03 ± 0.20 | 0.22 ± 0.03 | 1.14 ± 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillon, J.; Boudot, C.; Cohen, A.; Savrimoutou, S.; Rubio, S.; Milano, V.; Marchivie, M.; Azas, N.; Mullié, C.; Sonnet, P.; et al. Synthesis of 1H-3-{4-[(3-Dimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole and Evaluation of Its Antiprotozoal Activity. Molbank 2019, 2019, M1060. https://doi.org/10.3390/M1060
Guillon J, Boudot C, Cohen A, Savrimoutou S, Rubio S, Milano V, Marchivie M, Azas N, Mullié C, Sonnet P, et al. Synthesis of 1H-3-{4-[(3-Dimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole and Evaluation of Its Antiprotozoal Activity. Molbank. 2019; 2019(2):M1060. https://doi.org/10.3390/M1060
Chicago/Turabian StyleGuillon, Jean, Clotilde Boudot, Anita Cohen, Solène Savrimoutou, Sandra Rubio, Vittoria Milano, Mathieu Marchivie, Nadine Azas, Catherine Mullié, Pascal Sonnet, and et al. 2019. "Synthesis of 1H-3-{4-[(3-Dimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole and Evaluation of Its Antiprotozoal Activity" Molbank 2019, no. 2: M1060. https://doi.org/10.3390/M1060
APA StyleGuillon, J., Boudot, C., Cohen, A., Savrimoutou, S., Rubio, S., Milano, V., Marchivie, M., Azas, N., Mullié, C., Sonnet, P., & Courtioux, B. (2019). Synthesis of 1H-3-{4-[(3-Dimethylaminopropyl)aminomethyl]phenyl}-2-phenylindole and Evaluation of Its Antiprotozoal Activity. Molbank, 2019(2), M1060. https://doi.org/10.3390/M1060







