(S)-Ethyl 2-(tert-butoxycarbonylamino)-3-(2-iodo-4,5-methylenedioxyphenyl)propanoate
Abstract
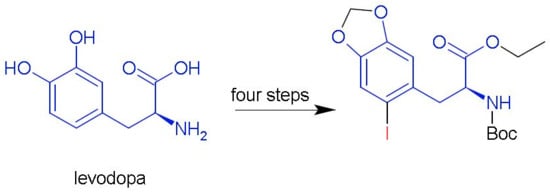
1. Introduction
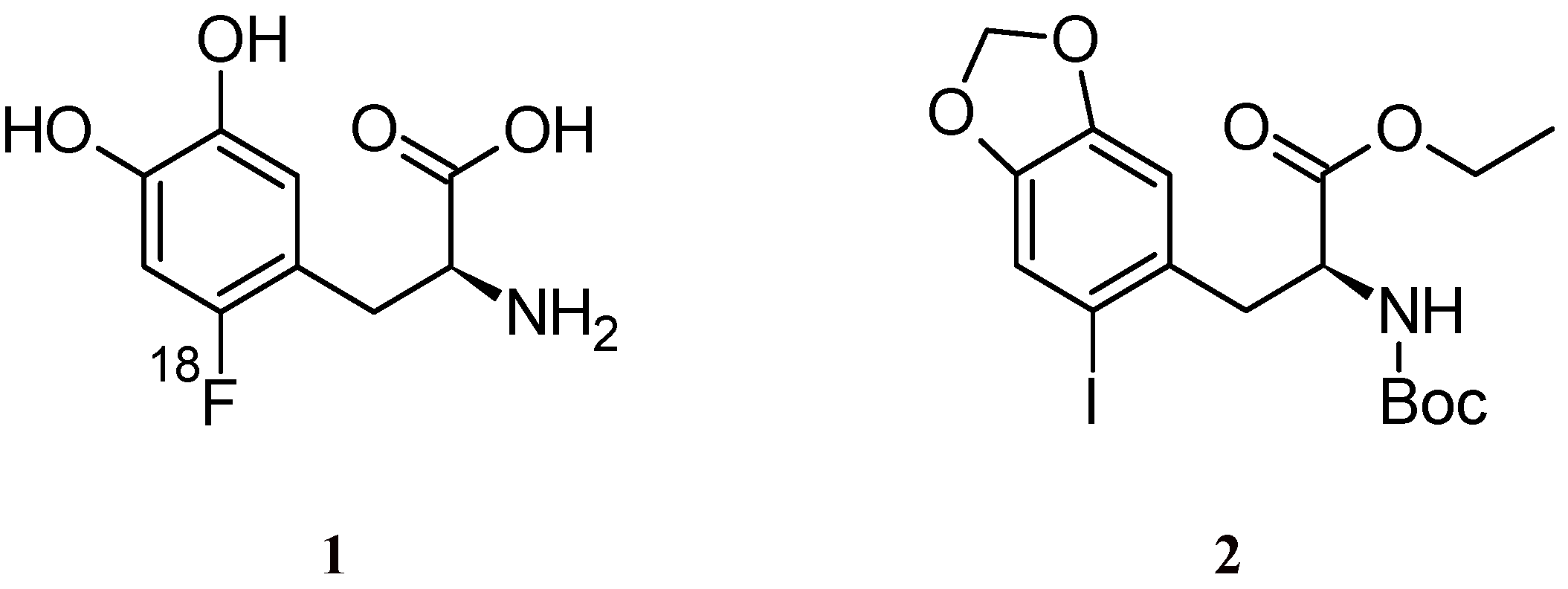
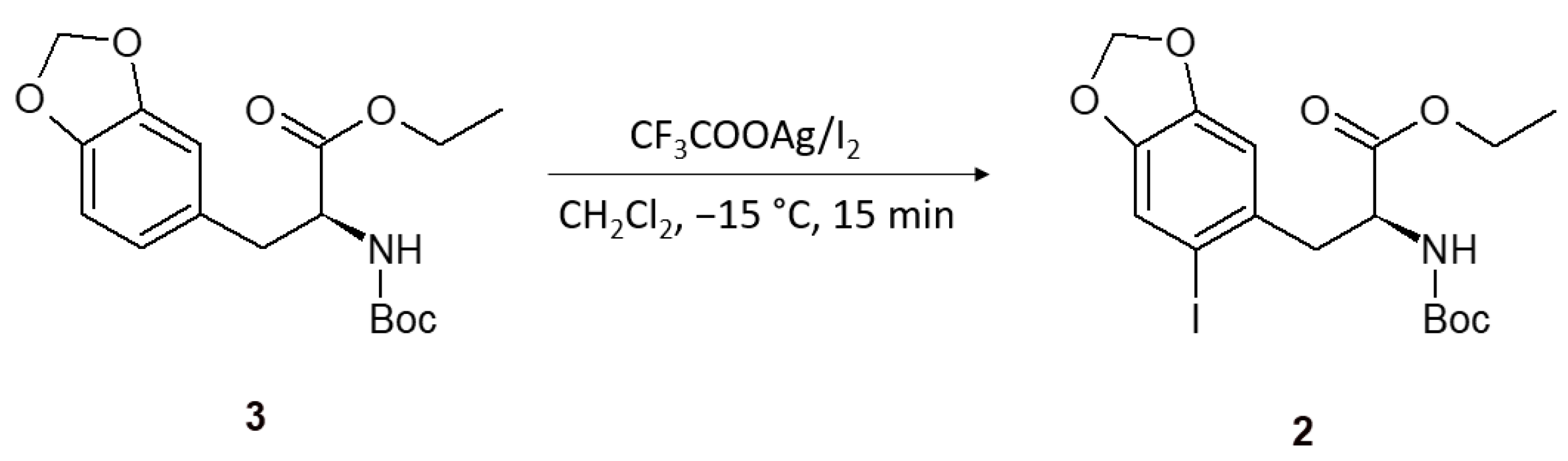
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. (S)-Ethyl 2-(tert-butoxycarbonylamino)-3-(2-iodo-4,5-methylenedioxyphenyl)propanoate (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Denora, N.; Lopedota, A.; de Candia, M.; Cellamare, S.; Degennaro, L.; Luisi, R.; Mele, A.; Tricarico, D.; Cutrignelli, A.; Laquintana, V.; et al. Pharmaceutical development of novel lactate-based 6-fluoro-l-DOPA formulations. Eur. J. Pharmac. Sci. 2017, 99, 361–368. [Google Scholar] [CrossRef]
- Trapani, A.; Tricarico, D.; Mele, A.; Maqoud, F.; Mandracchia, D.; Vitale, P.; Capriati, V.; Trapani, G.; Dimiccoli, V.; Tolomeo, A.; et al. A novel injectable formulation of 6-fluoro-l-DOPA imaging agent for diagnosis of neuroendocrine tumors and Parkinson’s disease. Int. J. Pharmac. 2017, 519, 304–313. [Google Scholar] [CrossRef]
- Seibyl, J.P.; Chen, W.; Silverman, D.H.S. 3,4-Dihydroxy-6-[18F]-fluoro-l-phenylalanine positron emission tomography in patients with central motor disorders and in evaluation of brain and other tumors. Semin. Nucl. Med. 2007, 37, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Lussey-Lepoutre, C.; Hindié, E.; Montravers, F.; Detour, J.; Ribeiro, M.-J.S.; Taïeb, D.; Imperiale, A. The current role of 18F-FDOPA PET for neuroendocrine tumor imaging. Med. Nucl. 2016, 40, 20–30. [Google Scholar] [CrossRef]
- Laakso, A.; Wallius, E.; Kajander, J.; Bergman, J.; Eskola, O.; Solin, O.; Ilonen, T.; Salokangas, R.K.R.; Syvälahti, E.; Hietala, J. Personality Traits and Striatal Dopamine Synthesis Capacity in Healthy Subjects. Am. J. Psychiatry 2003, 160, 904–910. [Google Scholar] [CrossRef] [PubMed]
- McGowan, S.; Lawrence, A.D.; Sales, T.; Quested, D.; Grasby, P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch. Gen. Psychiatry 2004, 61, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklin, H.F. Radiopharmaceuticals for drug development: Unites States regulatory perspective. Curr. Radiopharm. 2008, 1, 2–6. [Google Scholar] [CrossRef]
- Cole, E.L.; Stewart, M.N.; Littich, R.; Hoareau, R.; Scott, J.H. Radiosyntheses using fluorine-18: The art and science of late stage fluorination. Curr. Top. Med. Chem. 2014, 14, 875–900. [Google Scholar] [CrossRef] [PubMed]
- Coenen, H.H.; Ermert, J. Direct nucleophilic 18F-fluorination of electron-rich arenes: Present limits of no-carrier-added reactions. Curr. Radiopharm. 2010, 3, 163–173. [Google Scholar] [CrossRef]
- Tredwell, M.; Gouverneur, V. 18F labeling of arenes. Angew. Chem. Int. Ed. 2012, 51, 11426–11437. [Google Scholar] [CrossRef] [PubMed]
- Pimlott, S.L.; Sutherland, A. Molecular tracers for the PET and SPECT imaging of disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Chaly, T.; Diksic, M. High yield synthesis of 6-[18F]fluoro-l-Dopa by regioselective fluorination of protected l-Dopa with [18F]acetylhypofluorite. J. Nucl. Med. 1986, 27, 1896–1901. [Google Scholar] [PubMed]
- Edwards, R.; Wirth, T. [18F]6-Fluoro-3,4-dihydroxy-l-phenylalanine–recent modern syntheses for an elusive radiotracer. J. Label. Compd. Radiopharm. 2015, 58, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ichiishi, N.; Brooks, A.F.; Topczewski, J.J.; Rodnick, M.E.; Sanford, M.S.; Scott, P.J.H. Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett. 2014, 16, 3224–3227. [Google Scholar] [CrossRef] [PubMed]
- Makaravage, K.J.; Brooks, A.F.; Mossine, A.V.; Sanford, M.S.; Scott, P.J.H. Copper-mediated radiofluorination of arylstannanaes with [18F]KF. Org. Lett. 2016, 18, 5440–5443. [Google Scholar] [CrossRef]
- Tredwell, M.; Preshlock, S.M.; Taylor, N.J.; Gruber, S.; Huiban, M.; Passchier, J.; Mercier, J.; Génicot, C.; Gouverneur, V. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew. Chem. Int. Ed. Engl. 2014, 53, 7751–7755. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, C. Novel Preparation Method for Positron Medicine [18F]FDOPA and Intermediate Thereof. CN 107311877A, 3 November 2017. [Google Scholar]
- Chouhan, G.; Alper, H. Palladium-catalyzed carboxamidation reaction and aldol condensation reaction cascade: A facile approach to ring-fused isoquinolinones. Org. Lett. 2008, 10, 4987–4990. [Google Scholar] [CrossRef] [PubMed]
- Fier, P.S.; Hartwig, J.F. Copper-Mediated Fluorination of Aryl Iodides. J. Am. Chem. Soc. 2012, 134, 10795–10798. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lentini, G.; Cavalluzzi, M.M.; Degennaro, L.; Fracchiolla, G.; Perna, F.; Scilimati, A. (S)-Ethyl 2-(tert-butoxycarbonylamino)-3-(2-iodo-4,5-methylenedioxyphenyl)propanoate. Molbank 2019, 2019, M1049. https://doi.org/10.3390/M1049
Lentini G, Cavalluzzi MM, Degennaro L, Fracchiolla G, Perna F, Scilimati A. (S)-Ethyl 2-(tert-butoxycarbonylamino)-3-(2-iodo-4,5-methylenedioxyphenyl)propanoate. Molbank. 2019; 2019(1):M1049. https://doi.org/10.3390/M1049
Chicago/Turabian StyleLentini, Giovanni, Maria Maddalena Cavalluzzi, Leonardo Degennaro, Giuseppe Fracchiolla, Filippo Perna, and Antonio Scilimati. 2019. "(S)-Ethyl 2-(tert-butoxycarbonylamino)-3-(2-iodo-4,5-methylenedioxyphenyl)propanoate" Molbank 2019, no. 1: M1049. https://doi.org/10.3390/M1049
APA StyleLentini, G., Cavalluzzi, M. M., Degennaro, L., Fracchiolla, G., Perna, F., & Scilimati, A. (2019). (S)-Ethyl 2-(tert-butoxycarbonylamino)-3-(2-iodo-4,5-methylenedioxyphenyl)propanoate. Molbank, 2019(1), M1049. https://doi.org/10.3390/M1049