3-Chloro-5-(3-n-hexylthien-2-yl)-4H-1,2,6-thiadiazin-4-one
Abstract
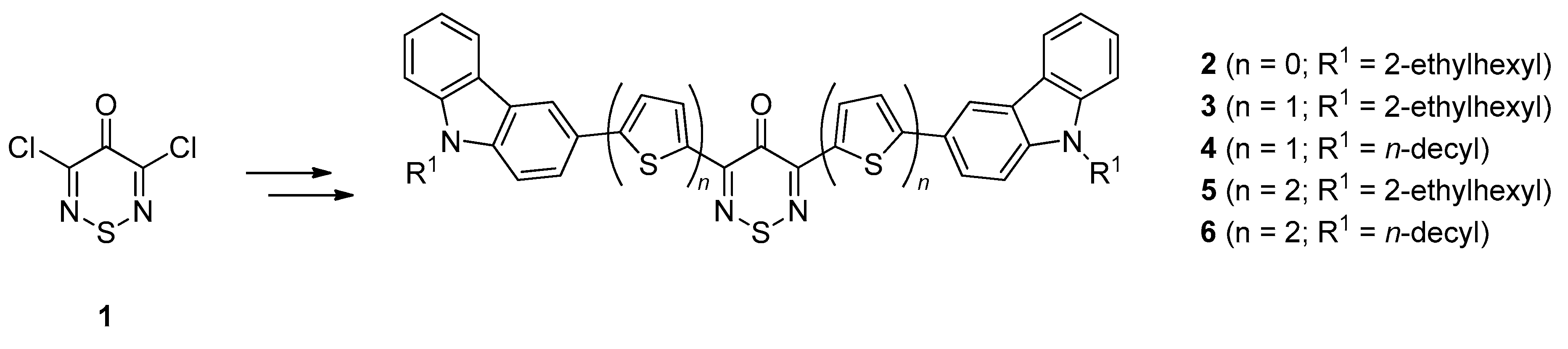
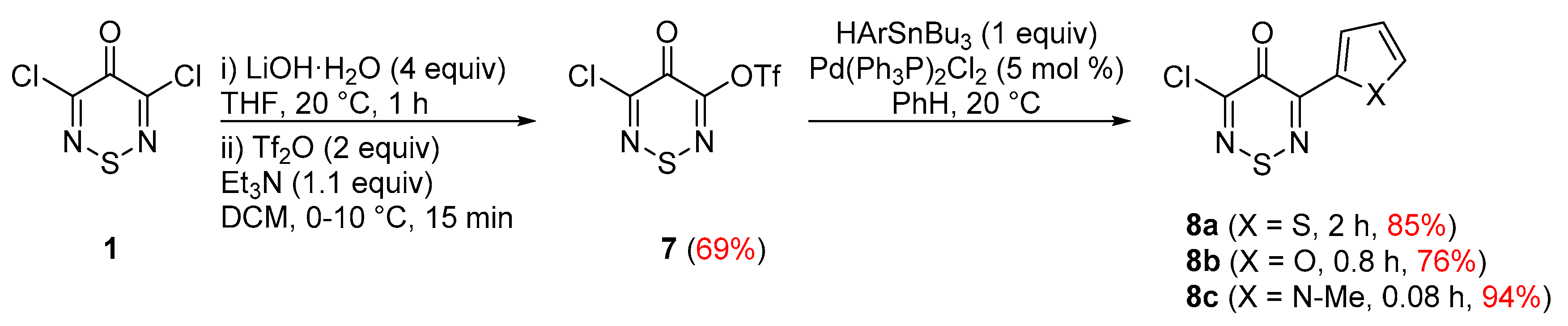
1. Introduction
2. Results and Discussion
3. Materials and Methods
3-Chloro-5-(3-n-hexylthien-2-yl)-4H-1,2,6-thiadiazin-4-one (9)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,097,594, 27 June 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-thio-3-chloro-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,100,281, 11 July 1978. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. 3-Chloro-5-(optionally substituted heterocycloxy)-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,143,138, 6 March 1979. [Google Scholar]
- Peake, C.J.; Harnish, W.N.; Davidson, B.L. Mono-5-substituted-3-chloro-4H-1,2,6-thiadiazin-4-one Antifungal Agents. U.S. Patent 4,201,780, 6 May 1980. [Google Scholar]
- Portnoy, R.C. Thiadiazinone plant disease control agents. U.S. Patent 4,497,807, 5 February 1985. [Google Scholar]
- Haddon, R.C.; Kaplan, M.L.; Marshall, J.H. Naphtho[1,8-cd:4,5-c′d′]bis[1,2,6]thiadiazine. A compound of ambiguous aromatic character. J. Am. Chem. Soc. 1978, 100, 1235–1239. [Google Scholar] [CrossRef]
- Gómez, T.; Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, Cyclohepta- and Cyclopentadithiazoles: New Materials and a Rich Heterocyclic Chemistry of Cyclic Enaminonitriles. Eur. J. Org. Chem. 2005, 2005, 5055–5066. [Google Scholar] [CrossRef]
- Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, new heterocyclic materials from cyclic Enaminonitriles. Chem. Commun. 2005, 3, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing Some New Life into an Old Topic: Chalcogen-Nitrogen π-Heterocycles as Electron Acceptors. Molecules 2013, 18, 9850–9900. [Google Scholar] [CrossRef] [PubMed]
- Cava, M.P.; Lakshmikantham, M.V.; Hoffmann, R.; Williams, R.M. R. B. Woodward’s unfinished symphony: Designing organic superconductors (1975–79). Tetrahedron 2011, 67, 6771–6797. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. Chem. Commun. 2000, 4, 303–304. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 7, 1089–1094. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Cyclisation chemistry of 4H-1,2,6-thiadiazines. J. Chem. Soc. Perkin Trans. 1 2000, 16, 2601–2607. [Google Scholar] [CrossRef]
- Theodorou, E.; Ioannidou, H.A.; Ioannou, T.A.; Kalogirou, A.S.; Constantinides, C.P.; Manoli, M.; Koutentis, P.A.; Hayes, S.C. Spectroscopic characterization of C-4 substituted 3,5-dichloro-4H-1,2,6-thiadiazines. RSC Adv. 2015, 51, 18471–18481. [Google Scholar] [CrossRef]
- Economopoulos, S.P.; Koutentis, P.A.; Ioannidou, H.A.; Choulis, S.A. Identifying potential candidates for donor–acceptor copolymers on a series of 4H-1,2,6-thiadiazines: An electrochemical approach. Electrochim. Acta 2013, 107, 448–453. [Google Scholar] [CrossRef]
- Hermerschmidt, F.; Kalogirou, A.S.; Min, J.; Zissimou, G.A.; Tuladhar, S.M.; Ameri, T.; Faber, H.; Itskos, G.; Choulis, S.A.; Anthopoulos, T.D.; et al. 4H-1,2,6-Thiadiazin-4-one-containing small molecule donors and additive effects on their performance in solution-processed organic solar cells. J. Mater. Chem. C 2015, 3, 2358–2365. [Google Scholar] [CrossRef]
- Chochos, C.L.; Kalogirou, A.S.; Ye, T.; Tatsi, E.; Katsouras, A.; Zissimou, G.A.; Gregoriou, V.G.; Avgeropoulos, A.; Koutentis, P.A. 4H-1,2,6-Thiadiazine-containing donor–acceptor conjugated polymers: Synthesis, optoelectronic characterization and their use in organic solar cells. J. Mater. Chem. C 2018, 6, 3658–3667. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Li, H.; Zhao, Z.; Lu, Z.; Huang, Y.; Xu, D. Density Functional Theory Investigations of D-A-D’ Structural Molecules as Donor Materials in Organic Solar Cell. Front. Chem. 2018, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Selective Stille coupling reactions of 3-chloro-5-halo(pseudohalo)-4H-1,2,6-thiadiazin-4-ones. Org. Lett. 2011, 13, 5886–5889. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Koutentis, P.A. 3-Chloro-5-(4-dodecylthiophen-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank 2012, 4, M782. [Google Scholar] [CrossRef]
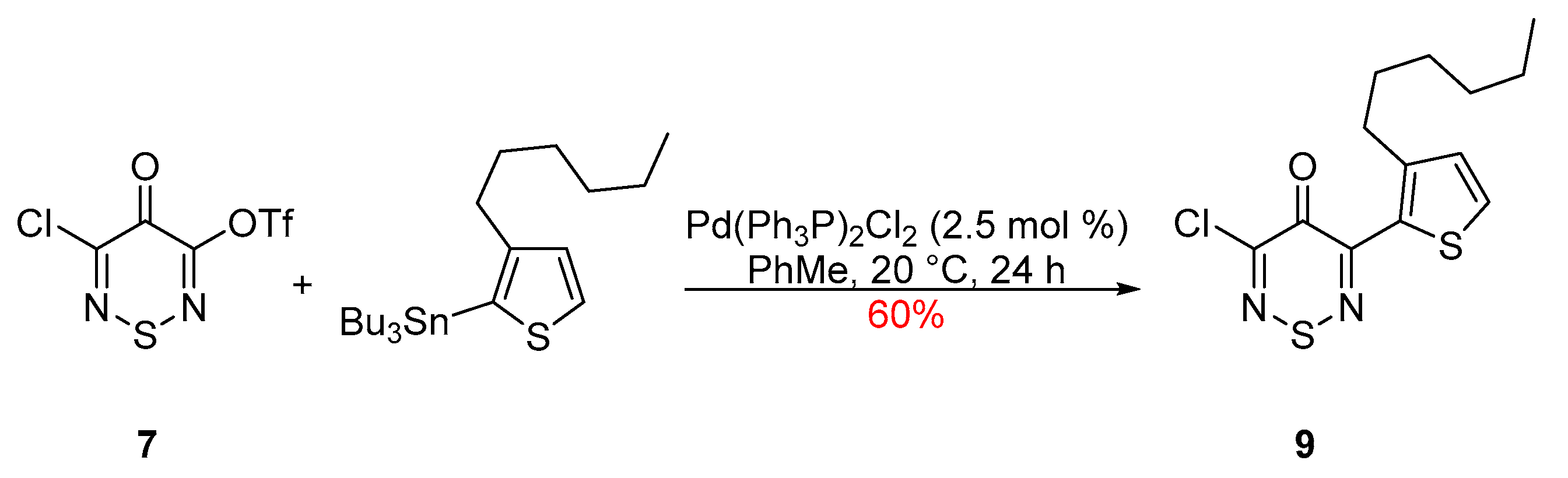
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. 3-Chloro-5-(3-n-hexylthien-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank 2019, 2019, M1043. https://doi.org/10.3390/M1043
Kalogirou AS, Koutentis PA. 3-Chloro-5-(3-n-hexylthien-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank. 2019; 2019(1):M1043. https://doi.org/10.3390/M1043
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2019. "3-Chloro-5-(3-n-hexylthien-2-yl)-4H-1,2,6-thiadiazin-4-one" Molbank 2019, no. 1: M1043. https://doi.org/10.3390/M1043
APA StyleKalogirou, A. S., & Koutentis, P. A. (2019). 3-Chloro-5-(3-n-hexylthien-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank, 2019(1), M1043. https://doi.org/10.3390/M1043





