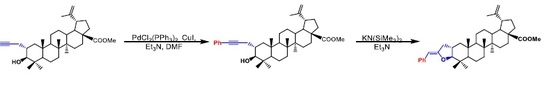
Effective Synthesis of a Novel Tetrahydrofuran Containing Triterpenoid: 5′(Z)-Benzylidene-tetrahydrofurano[3,2-b]lup-20(29)-en-28-oate
Abstract
1. Introduction
2. Results
3. Materials and Methods
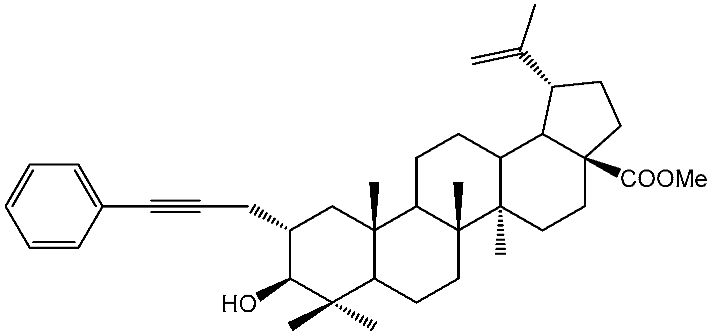
3.1. 2a-Phenylpropynyl-3β-hydroxylup-20(29)-en-28-oate (5)
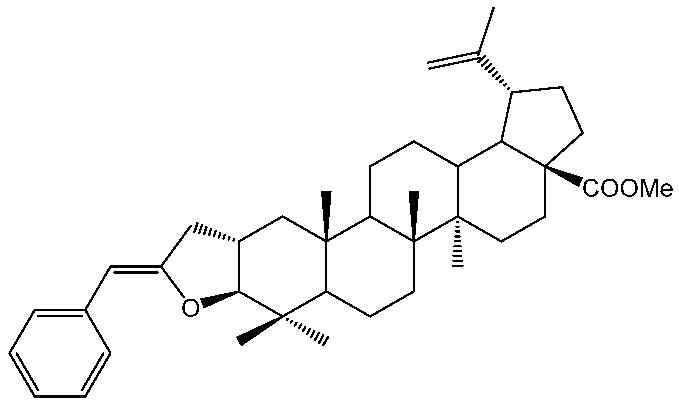
3.2. 5′(Z)-Benzylidene-tetrahydrofuran[3,2-b]lup-20(29)-en-28-oate (6)
3.3. 2a-Phenylaceton-3β-hydroxylup-20(29)en-28-oate (7)

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Sarek, J.; Kvasnica, M.; Vlk, M.; Urban, M.; Dzubak, P.; Hajduch, M. The Potential of Triterpenoids in the Treatment of Melanoma. In Research on Melanoma—A Glimpse into Current Directions and Future Trends; Kvasnica, M., Ed.; InTech: Rijeka, Croatia, 2011; Volume 7, pp. 125–158. [Google Scholar]
- Mukherjee, R.; Kumar, V.; Srivastava, S.K.; Agarwal, S.K.; Burman, A.C. Betulinic acid derivatives as anticancer agents: Structure activity relationship. Anticancer Agents: Med. Chem. 2006, 6, 271–279. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Kvasnica, M.; Urban, M.; Dickinson, N.J.; Sarek, J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. Rep. 2015, 32, 1303–1330. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Haavikko, R.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Alakurtti, S.; Moreira, V.M.; Yli-Kauhaluoma, J.; Moilanen, E. Betulin Derivatives Effectively Suppress Inflammation in Vitro and in Vivo. J. Nat. Prod. 2016, 79, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Haavikko, R.; Nasereddin, A.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C.L.; Yli-Kauhaluoma, J. Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes. Medchemcomm 2014, 5, 445–451. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, N.; Aggarwal, P.; Sanna, V.K.; Singh, A.T.; Jaggi, M.; Joshi, N.; Sharma, P.K.; Irchhaiya, R.; Burman, A.C. Synthesis and cytotoxic activity of heterocyclic ring-substituted betulinic acid derivatives. Bioorganic Med. Chem. Lett. 2008, 18, 5058–5062. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Sarek, J.; Kvasnica, M.; Tislerova, I.; Hajduch, M. Triterpenoid Pyrazines and Benzopyrazines With Cytotoxic Activity. J. Nat. Prod. 2007, 70, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Bellur, E.; Freifeld, I.; Böttcher, D.; Bornscheuer, U.T.; Langer, P. Synthesis of (tetrahydrofuran-2-yl)acetates based on a ′cyclization/hydrogenation/enzymatic kinetic resolution′ strategy. Tetrahedron 2006, 62, 7132–7139. [Google Scholar] [CrossRef]
- Bellur, E.; Langer, P. Synthesis of Functionalized 2-Alkylidene-tetrahydrofurans Based on a [3 + 2] Cyclization/Bromination/Palladium(0) Cross-Coupling Strategy. Eur. J. Org. Chem. 2005, 2005, 4815–4828. [Google Scholar] [CrossRef]
- Zeiss, H.J. Recent advances in the stereoselective synthesis of l-phosphinothricin. Pestic. Sci. 1994, 41, 269–277. [Google Scholar] [CrossRef]
- Lorente, A.; Lamariano-Merketegi, J.; Albericio, F.; Álvarez, M. Tetrahydrofuran-Containing Macrolides: A Fascinating Gift from the Deep Sea. Chem. Rev. 2013, 113, 4567–4610. [Google Scholar] [CrossRef] [PubMed]
- Gubaidullin, R.R.; Khalitova, R.R.; Galimshina, Z.R.; Spivak, A.Y. Synthesis of novel [3,2-b] furan-fused pentacyclic triterpenoids via gold—Catalyzed intramolecular heterocyclization of 2-alkynyl-3-oxotriterpene acids. Tetrahedron 2018. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Gubaidullin, R.R.; Galimshina, Z.R.; Nedopekina, D.A.; Odinokov, V.N. Effective synthesis of novel C(2)-propargyl derivatives of betulinic and ursolic acids and their conjugation with β-d-glucopyranoside azides via click chemistry. Tetrahedron 2016, 72. [Google Scholar] [CrossRef]
- Luo, F.T.; Schreuder, I.; Wang, R.T. Intramolecular oxypalladation and cross-coupling of acetylenic alkoxides. J. Org. Chem. 1992, 57, 2213–2215. [Google Scholar] [CrossRef]
- Riediker, M.; Schwartz, J. Mercury(II)-induced cyclization of acetylenic alcohols: A new route to enol ethers and substituted enol ethers. J. Am. Chem. Soc. 1982, 104, 5842–5844. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Chen, Z.; Nguyen, v.T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.Z. A Concise Semi-Synthetic Approach to Betulinic Acid from Betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubaidullin, R.; Nedopekina, D.; Evstifeeva, R.; Prochukhan, Y. Effective Synthesis of a Novel Tetrahydrofuran Containing Triterpenoid: 5′(Z)-Benzylidene-tetrahydrofurano[3,2-b]lup-20(29)-en-28-oate. Molbank 2019, 2019, M1042. https://doi.org/10.3390/M1042
Gubaidullin R, Nedopekina D, Evstifeeva R, Prochukhan Y. Effective Synthesis of a Novel Tetrahydrofuran Containing Triterpenoid: 5′(Z)-Benzylidene-tetrahydrofurano[3,2-b]lup-20(29)-en-28-oate. Molbank. 2019; 2019(1):M1042. https://doi.org/10.3390/M1042
Chicago/Turabian StyleGubaidullin, Rinat, Darya Nedopekina, Regina Evstifeeva, and Yurij Prochukhan. 2019. "Effective Synthesis of a Novel Tetrahydrofuran Containing Triterpenoid: 5′(Z)-Benzylidene-tetrahydrofurano[3,2-b]lup-20(29)-en-28-oate" Molbank 2019, no. 1: M1042. https://doi.org/10.3390/M1042
APA StyleGubaidullin, R., Nedopekina, D., Evstifeeva, R., & Prochukhan, Y. (2019). Effective Synthesis of a Novel Tetrahydrofuran Containing Triterpenoid: 5′(Z)-Benzylidene-tetrahydrofurano[3,2-b]lup-20(29)-en-28-oate. Molbank, 2019(1), M1042. https://doi.org/10.3390/M1042