5-(6-Hydroxy-6-methyl-5-oxoheptan-2-yl)-2-methyl Phenyl Acetate
Abstract
1. Introduction
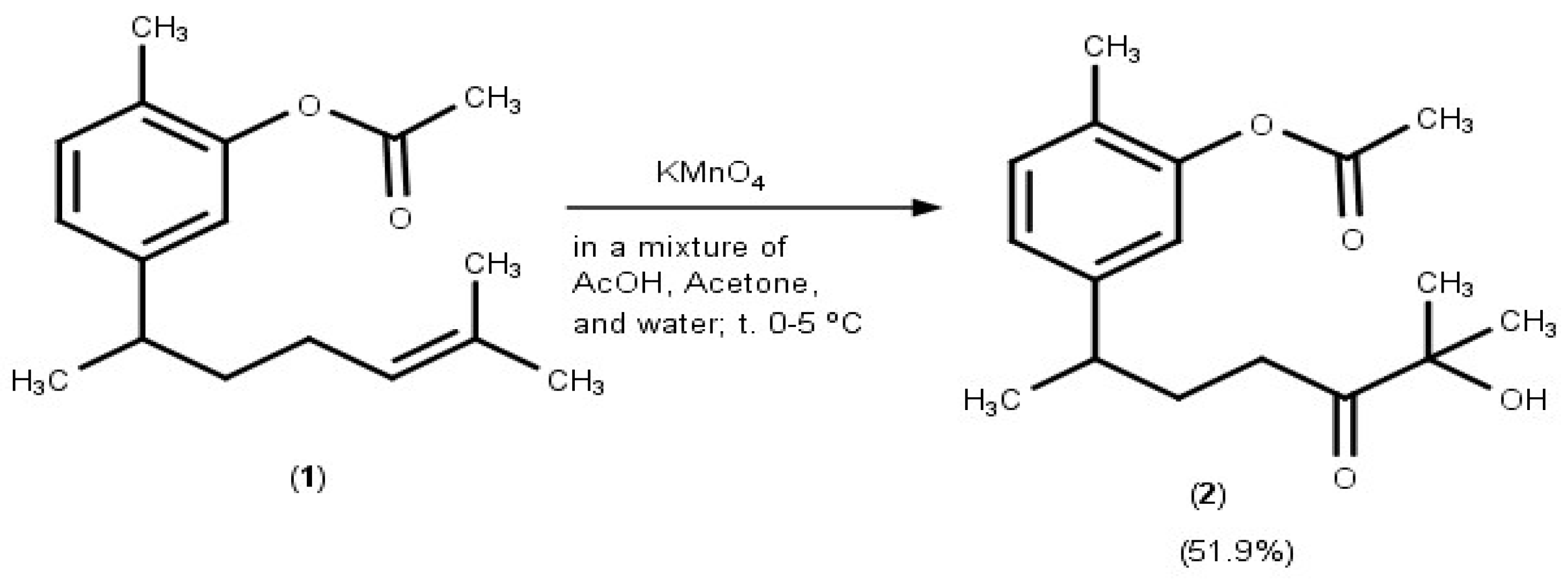
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of the Title Compound 2
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nurcholis, W.; Munshif, A.A.; Ambarsari, L. Xanthorrhizol contents, α-glucosidase inhibition, and cytotoxic activities in ethyl acetate fraction of Curcuma zanthorrhiza accessions from Indonesia. Rev. Bras. Farmacogn. 2018, 28, 44–49. [Google Scholar] [CrossRef]
- Chung, W.Y.; Park, J.H.; Kim, M.J.; Kim, H.O.; Hwang, J.K.; Lee, S.K.; Park, K.K. Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-κB. Carcinogenesis 2007, 28, 1224–1231. [Google Scholar] [PubMed]
- Kang, Y.J.; Park, K.K.; Chung, W.Y.; Hwang, J.K.; Lee, S.K. Xanthorrhizol, a natural sesquiterpenoid, induces apoptosis and growth arrest in HCT116 human colon cancer cells. J. Pharmacol. Sci. 2009, 111, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, C.; Song, Y.; Hwang, J.K. Antihyperglycemic and Anti-Inflammatory Effects of Standardized Curcuma xanthorrhiza Roxb. Extract and Its Active Compound Xanthorrhizol in High-Fat Diet-Induced Obese Mice. Evid. Based Complement. Alternat. Med. 2014. [Google Scholar] [CrossRef]
- Choi, M.; Kim, S.H.; Chung, W.Y.; Hwang, J.K.; Park, K.K. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem. Biophys. Res. Commun. 2004, 326, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.H.; Azimahtol, H.L.P.; Abdullah, N.R. Xanthorrhizol Exhibits Antiproliferative Activity on MCF-7 Breast Cancer Cells via Apoptosis Induction. Anticancer Res. 2006, 26, 4527–4534. [Google Scholar] [PubMed]
- Rukayadi, Y.; Yong, D.; Hwang, J.K. In vitro anticandidal activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. J. Antimicrob. Chemother. 2006, 57, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Shim, J.S.; Rukayadi, Y.; Hwang, J.K. Antibacterial Activity of Xanthorrhizol Isolated from Curcuma xanthorrhiza Roxb. against Foodborne Pathogens. J. Food Prot. 2008, 71, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- Oon, S.F.; Nallappan, M.; Tee, T.T.; Shohaimi, S.; Kassim, N.K.; Sa’ariwijaya, M.S.F.; Cheah, Y.H. Xanthorrhizol: A review of its pharmacological activities and anticancer properties. Cancer Cell Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.I.; Delgado, G.; Villarreal, M.L. New bioactive derivatives of xanthorrhizol. Rev. Soc. Quím. Mex. 2001, 45, 56–59. [Google Scholar]
- Sirat, H.M.; Hong, N.M.; Jauri, M.H. Chemistry of xanthorrhizol: Synthesis of several bisabolane sesquiterpenoids from xanthorrhizol. Tetrahedron Lett. 2007, 48, 457–460. [Google Scholar] [CrossRef]
- Dash, S.; Patel, S.; Mishra, B.K. Oxidation by permanganate: Synthetic and mechanistic aspects. Tetrahedron 2009, 65, 707–739. [Google Scholar] [CrossRef]
- Lugemwa, F.N.; Shaikh, K.; Hochstedt, E. Facile and Efficient Acetylation of Primary Alcohols and Phenols with Acetic Anhydride Catalyzed by Dried Sodium Bicarbonate. Catalysts 2013, 3, 954–965. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- MarvinSketch 17.17.0. Chemaxon Ltd. 1998–2017. Available online: http://www.chemaxon.com (accessed on 20 November 2018).
- Alimuddin, A.H.; Mardjan, M.I.D.; Chairil, S.M.; Anwar4, A.; Sholikhah, E.N. Synthesis 7-Hydroxy-3’,4’-dimethoxyisoflavon from Eugenol. Indones. J. Chem. 2011, 11, 163–168. [Google Scholar]

| Proton | H-1 | H-2 | H-3 | H-4 | H-6’ | H-7 | H-1” | H-2’ | H-3 Ar | H-4 Ar | H-6 Ar | H-O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon | ppm | 1.25 | 2.67 | 1.82 & 1.91 | 2.41 | 1.26 | 1.27 | 2.31 | 2.14 | 6.95 | 7.14 | 6.79 | 3.71 |
| C-1 | 22.7 | DB * | α | β | γ | ||||||||
| C-2 | 38.9 | α | DB * | α | β | γ | β | ||||||
| C-3 | 31.8 | β | α | DB * | α | ||||||||
| C-4 | 33.6 | α | DB * | ||||||||||
| C-5 | 214.6 | ||||||||||||
| C-6 | 76.3 | α | α | α | |||||||||
| C-6’ | 26.6 | DB * | β | ||||||||||
| C-7 | 26.5 | DB * | β | ||||||||||
| C-1’ | 169.4 | α | |||||||||||
| C-1” | 21.0 | DB * | |||||||||||
| C-2’ | 15.9 | DB * | γ | ||||||||||
| C-1 Ar | 149.5 | β | γ | α | |||||||||
| C-2 Ar | 127.9 | α | α | β | |||||||||
| C-3 Ar | 131.3 | β | DB * | ||||||||||
| C-4 Ar | 124.7 | β | DB * | β | |||||||||
| C-5 Ar | 145.4 | β | α | β | α | α | |||||||
| C-6 Ar | 120.6 | β | γ | DB * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, M.D.; Kusumaningrum, S.; Hayun, H. 5-(6-Hydroxy-6-methyl-5-oxoheptan-2-yl)-2-methyl Phenyl Acetate. Molbank 2019, 2019, M1041. https://doi.org/10.3390/M1041
Rahayu MD, Kusumaningrum S, Hayun H. 5-(6-Hydroxy-6-methyl-5-oxoheptan-2-yl)-2-methyl Phenyl Acetate. Molbank. 2019; 2019(1):M1041. https://doi.org/10.3390/M1041
Chicago/Turabian StyleRahayu, Maya Damayanti, Susi Kusumaningrum, and Hayun Hayun. 2019. "5-(6-Hydroxy-6-methyl-5-oxoheptan-2-yl)-2-methyl Phenyl Acetate" Molbank 2019, no. 1: M1041. https://doi.org/10.3390/M1041
APA StyleRahayu, M. D., Kusumaningrum, S., & Hayun, H. (2019). 5-(6-Hydroxy-6-methyl-5-oxoheptan-2-yl)-2-methyl Phenyl Acetate. Molbank, 2019(1), M1041. https://doi.org/10.3390/M1041





