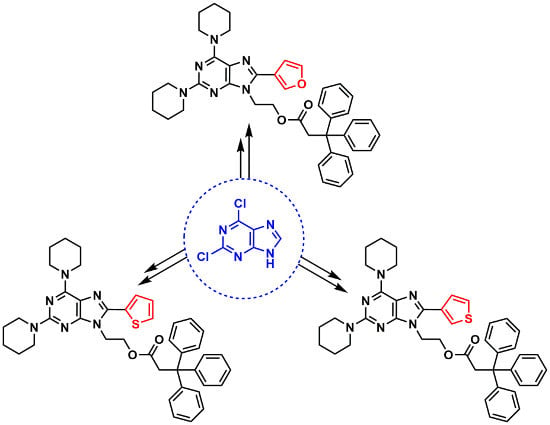
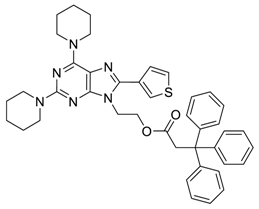
Purine-Furan and Purine-Thiophene Conjugates
Abstract
1. Introduction
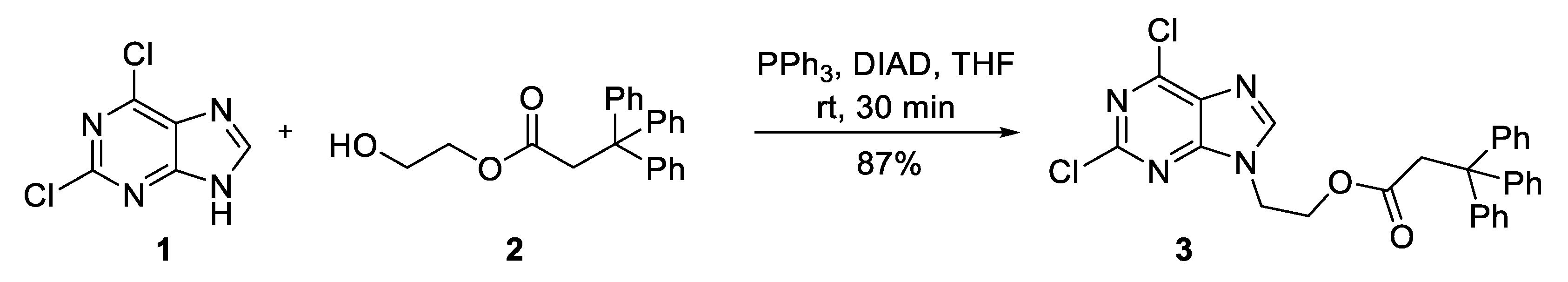
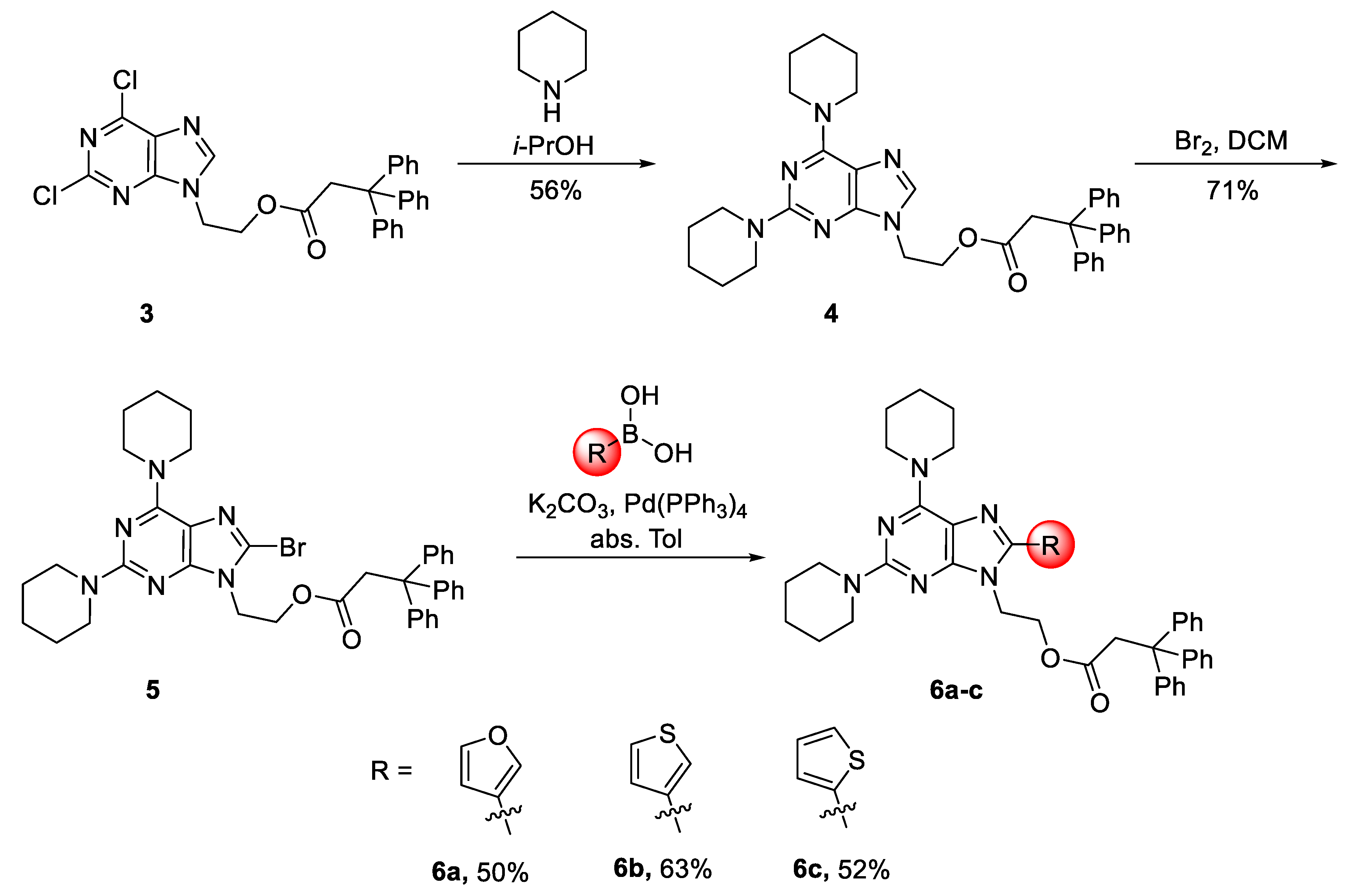
2. Results and Discussion
3. Materials and Methods

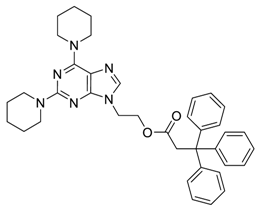
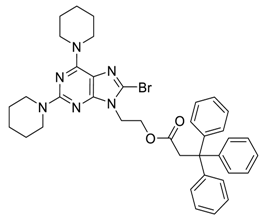
General Procedure for the Suzuki–Miyaura Reaction
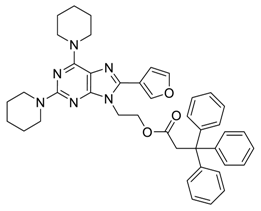

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Cohn, P.; Eom, S.H.; Abboud, K.A.; Castellano, R.K.; Xue, J. Ultraviolet-Violet Electroluminescence from Highly Fluorescent Purines. J. Mater. Chem. C 2013, 1, 2867–2874. [Google Scholar] [CrossRef]
- Greco, N.J.; Tor, Y. Furan Decorated Nucleoside Analogues as Fluorescent Probes: Synthesis, Photophysical Evaluation and Site-Specific Incorporation. Tetrahedron 2007, 63, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Luedtke, N.W. Site-Specific Control of N7-Metal Coordination in DNA by a Fluorescent Purine Derivative. Chem. Eur. J. 2012, 18, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Collier, G.S.; Brown, L.A.; Boone, E.S.; Kaushal, M.; Ericson, M.N.; Walter, M.G.; Long, B.K.; Kilbey, S.M. Linking Design and Properties of Purine-Based Donor-Acceptor Chromophores as Optoelectronic Materials. J. Mater. Chem. C 2017, 5, 6891–6898. [Google Scholar] [CrossRef]
- Traskovskis, K.; Mihailovs, I.; Tokmakovs, A.; Jurgis, A.; Kokars, V.; Rutkis, M. Triphenyl Moieties as Building Blocks for Obtaining Molecular Glasses with Nonlinear Optical Activity. J. Mater. Chem. 2012, 22, 11268–11276. [Google Scholar] [CrossRef]
- Ozola, V.; Persson, T.; Gronowitz, S.; Hӧrnfeldt, A.-B. On the Syntheses of 8-Heteroaryl-Substituted 9-(β-d-Ribofuranosyl)-2,6-Diaminopurines through Pd-Catalyzed Coupling in the Presence of Cupric Oxide. J. Heterocycl. Chem. 1995, 32, 863–866. [Google Scholar] [CrossRef]
- Sedláček, O.; Břehová, P.; Pohl, R.; Holý, A.; Janeba, Z. The Synthesis of the 8- C-Substituted 2,6-Diamino-9-[2-(phosphonomethoxy)ethyl]purine (PMEDAP) Derivatives by Diverse Cross-Coupling Reactions. Can. J. Chem. 2011, 89, 488–498. [Google Scholar] [CrossRef]
- Vaňková, B.; Krchňák, V.; Soural, M.; Hlavác, J. Direct C-H Arylation of Purine on Solid Phase and Its Use for Chemical Libraries Synthesis. ACS Comb. Sci. 2011, 13, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Mikhnenko, O.V.; Blom, P.W.M.; Nguyen, T.-Q. Exciton Diffusion in Organic Semiconductors. Energy Environ. Sci. 2015, 8, 1867–1888. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sasaki, M.; Ohsawa, N.; Yasuda, K.; Kotani, M. Fluorescence and Its Density-Dependent Quenching of a Sub-Monolayer Film of Methylene Blue Prepared by Dip Coating. J. Phys. Chem. C 2007, 111, 268–271. [Google Scholar] [CrossRef]
- Penzkofer, A.; Lu, Y. Fluorescence Quenching of Rhodamine 6G in Methanol at High Concentration. Chem. Phys. 1986, 103, 399–405. [Google Scholar] [CrossRef]
- Musser, A.J.; Rajendran, S.K.; Georgiou, K.; Gai, L.; Grant, R.T.; Shen, Z.; Cavazzini, M.; Ruseckas, A.; Turnbull, G.A.; Samuel, I.D.W.; et al. Intermolecular States in Organic Dye Dispersions: Excimers: Vs. aggregates. J. Mater. Chem. C 2017, 5, 8380–8389. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and Exciplexes of Conjugated Polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Currie, M.J.; Mapel, J.K.; Heidel, T.D.; Goffri, S.; Baldo, M.A. High-Efficiency Organic Solar Concentrators for Photovoltaics. Science 2008, 321, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.C.; Lin, T.N.; Lin, H.T.; Talite, M.J.; Tzeng, T.T.; Hsu, C.L.; Chiu, K.P.; Lin, C.A.J.; Shen, J.L.; Yuan, C.T. A Facile and Low-Cost Method to Enhance the Internal Quantum Yield and External Light-Extraction Efficiency for Flexible Light-Emitting Carbon-Dot Films. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Banal, J.L.; White, J.M.; Ghiggino, K.P.; Wong, W.W.H. Concentrating Aggregation-Induced Fluorescence in Planar Waveguides: A Proof-of-Principle. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
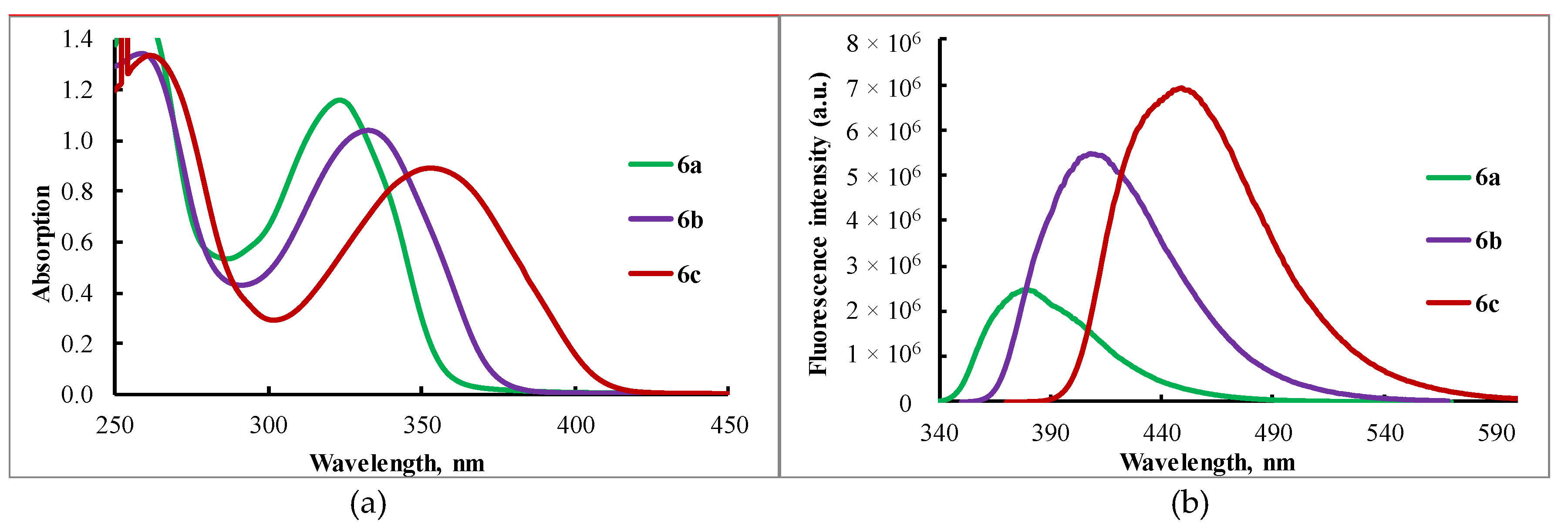
| Compound | Solvent/Thin Layer | λabs, nm | log ε | λem, nm | QY |
|---|---|---|---|---|---|
| 6a | DCM | 323 | 4.4 | 380 | 0.18 |
| Thin layer | 326 | - | 382 | <0.01 | |
| 6b | DCM | 333 | 4.3 | 408 | 0.60 |
| Thin layer | 336 | - | 415 | 0.04 | |
| 6c | DCM | 353 | 4.3 | 449 | 0.88 |
| Thin layer | 356 | - | 445 | 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapilinskis, Z.; Novosjolova, I.; Turks, M. Purine-Furan and Purine-Thiophene Conjugates. Molbank 2018, 2018, M1024. https://doi.org/10.3390/M1024
Kapilinskis Z, Novosjolova I, Turks M. Purine-Furan and Purine-Thiophene Conjugates. Molbank. 2018; 2018(4):M1024. https://doi.org/10.3390/M1024
Chicago/Turabian StyleKapilinskis, Zigfrīds, Irina Novosjolova, and Māris Turks. 2018. "Purine-Furan and Purine-Thiophene Conjugates" Molbank 2018, no. 4: M1024. https://doi.org/10.3390/M1024
APA StyleKapilinskis, Z., Novosjolova, I., & Turks, M. (2018). Purine-Furan and Purine-Thiophene Conjugates. Molbank, 2018(4), M1024. https://doi.org/10.3390/M1024