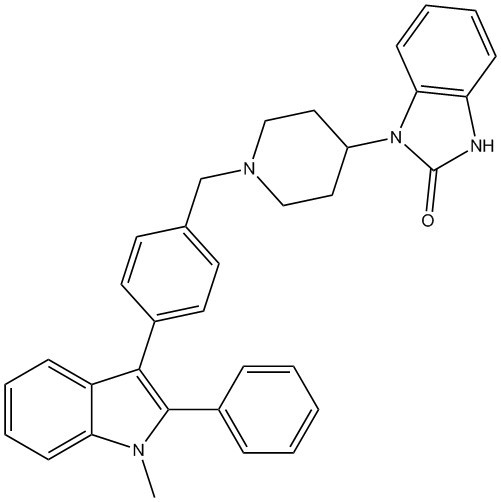
1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole
Abstract
1. Introduction
2. Results and Discussion
2.1. 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole
2.2. Cytotoxic Activity
3. Materials and Methods
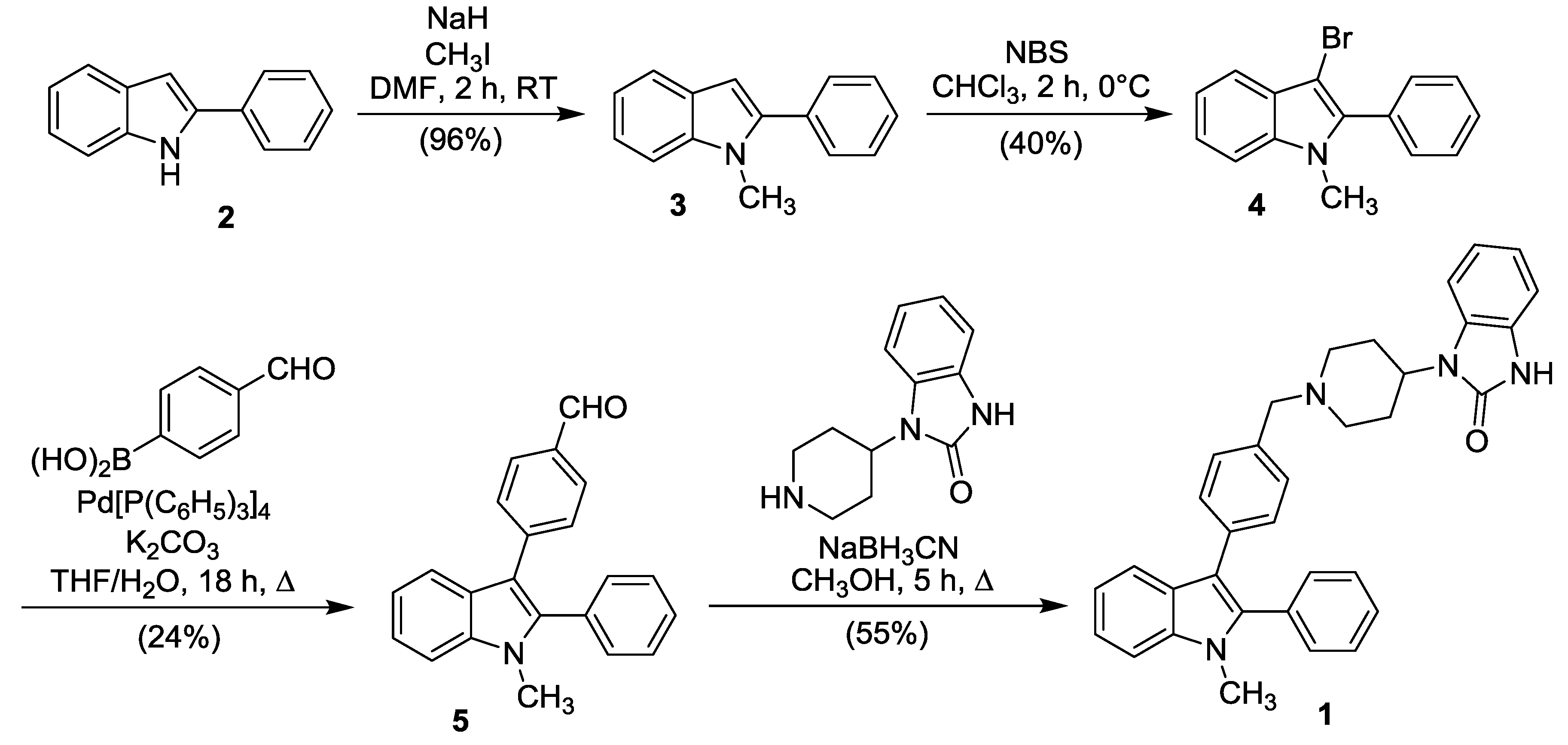
3.1. 3-(4-Formylphenyl)-1-methyl-2-phenyl-indole (5)
3.2. 1-M.ethyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole (1)
3.3. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shafakat Alia, N.A.; Darab, B.A.; Pradhana, V.; Farooquia, M. Chemistry and Biology of indoles and Indazoles: A mini-review. Mini-Rev. Med. Chem. 2013, 13, 1792–1800. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Ha Choi, E. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Sahoo, U.; Sethy, S.; Kumar, H.K.S.; Banerjee, M. Indole: The molecule of diverse biological activities. Asian J. Pharm. Clin. Res. 2012, 5, 1–6. [Google Scholar]
- Sharma, V.; Kumar, V.; Pathak, D. Biological Importance of the Indole Nucleus in Recent Years: A Comprehensive Review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Johansson, H.; Bøgeløv Jørgensen, T.; Gloriam, D.E.; Braüner-Osborne, H.; Sejer Pedersen, D. 3-Substituted 2-phenyl-indoles: Privileged structures for medicinal chemistry. RSC Adv. 2013, 3, 945–960. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, Q.; Yang, G.F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Sherer, C.; Snape, T.J. Heterocyclic scaffolds as promising anticancer agents against tumours of the central nervous system: Exploring the scope of indole and carbazole derivatives. Eur. J. Med. Chem. 2015, 97, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sakr, W.A.; Rahman, K.M. Anticancer properties of indole compounds: Mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets 2010, 11, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelian, B.; Benkendorff, K.; Johnston, M.R.; Abbott, C.A. Purified Brominated Indole Derivatives from Dicathais orbita Induce Apoptosis and Cell Cycle Arrest in Colorectal Cancer Cell Lines. Mar. Drugs. 2013, 11, 3802–3822. [Google Scholar] [CrossRef] [PubMed]
- Pelz, N.F.; Bian, Z.; Zhao, B.; Shaw, S.; Tarr, J.C.; Belmar, J.; Gregg, C.; Camper, D.V.; Goodwin, C.M.; Arnold, A.L.; et al. Discovery of 2-Indole-acylsulfonamide Myeloid Cell Leukemia 1 (Mcl-1) Inhibitors Using Fragment-Based Methods. J. Med. Chem. 2016, 59, 2054–2066. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Weng, J.R.; Chiu, C.F.; Wu, C.Y.; Yeh, S.P.; Sargeant, A.M.; Lin, P.H.; Liao, Y.M. OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis. Biochem. Pharmacol. 2013, 86, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Cruz-Lopez, O.; Cara, C.L.; Preti, D.; Tabrizi, M.A.; Balzarini, J.; Hamel, E.; Fabbri, E.; et al. Discovery of 8-methoxypyrazino[1,2-a]indole as a New Potent Antiproliferative Agent Against Human Leukemia K562 Cells. A Structure-Activity Relationship Study. Lett. Drug Des. Discov. 2009, 6, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Sheikhrezaei, Z.; Heydari, P.; Farsinezhad, A.; Fatemi, A.; Falahati-Pour, S.K.; Darakhshan, S.; Karimabad, M.N.; Darekordi, A.; Khorramdelazad, H.; Hassanshahi, G. A New Indole Derivative Decreased SALL4 Gene Expression in Acute Promyelocytic Leukemia Cell Line (NB4). Iran Biomed. J. 2018, 22, 99–106. [Google Scholar] [PubMed]
- Guillon, J.; Vincenzi, M.; Pinaud, N.; Ronga, L.; Rossi, F.; Savrimoutou, S.; Moreau, S.; Desplat, V.; Marchivie, M. Synthesis and Crystal Structure of 3-{4-[(4-(2-Oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Struct. Chem. Crystallogr. Commun. 2016, 2, 1. [Google Scholar]
- Barraja, P.; Diana, P.; Carbone, A.; Cirrincione, G. Nucleophilic reactions in the indole series: Displacement of bromine under phase transfer catalysis. Tetrahedron 2008, 64, 11625–11631. [Google Scholar] [CrossRef]
- Kim, D.; Kang, M.-S.; Song, K.; Kang, S.O.; Ko, J. Molecular engineering of organic sensitizers containing indole moiety for dye-sensitized solar cells. Tetrahedron 2008, 64, 10417–10424. [Google Scholar] [CrossRef]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; et al. Synthesis and evaluation of the cytotoxic activity of novel ethyl 4-[4-(4-substitutedpiperidin-1-yl)]benzyl-phenylpyrrolo[1,2-a]quinoxaline-carboxylate derivatives in myeloid and lymphoid leukemia cell lines. Eur. J. Med. Chem. 2016, 113, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; et al. Synthesis and Antiproliferative Effect of Ethyl 4-[4-(4-Substituted Piperidin-1-yl)]benzylpyrrolo[1,2-a]quinoxalinecarboxylate Derivatives on Human Leukemia Cells. ChemMedChem 2017, 12, 940–953. [Google Scholar] [CrossRef] [PubMed]
| Compound | IC50 values (μM) [a] | |||||
|---|---|---|---|---|---|---|
| K562 | U937 | HL60 | Jurkat | U266 | PBMNC + PHA | |
| 1 | 8 | >50 | 12 | 6 | >50 | >50 |
| A6730 | 17 | 8 | 5.5 | 3.5 | n.d. [b] | >50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillon, J.; Savrimoutou, S.; Rubio, S.; Desplat, V. 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Molbank 2018, 2018, M1023. https://doi.org/10.3390/M1023
Guillon J, Savrimoutou S, Rubio S, Desplat V. 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Molbank. 2018; 2018(4):M1023. https://doi.org/10.3390/M1023
Chicago/Turabian StyleGuillon, Jean, Solène Savrimoutou, Sandra Rubio, and Vanessa Desplat. 2018. "1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole" Molbank 2018, no. 4: M1023. https://doi.org/10.3390/M1023
APA StyleGuillon, J., Savrimoutou, S., Rubio, S., & Desplat, V. (2018). 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Molbank, 2018(4), M1023. https://doi.org/10.3390/M1023