Abstract
1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine (3) has been successfully synthesized by reacting p-toluic hydrazide (1) and glycine (2) via the polyphosphoric acid condensation route. The course of the reaction was found to be high yielding (87%) and the title compound was spectroscopically characterized by UV-Vis, FTIR, DSC, 13C/1H-NMR, and sass spectrometric techniques.
1. Introduction
Among the preeminent heterocyclic compounds, oxadiazole structures have been demonstrated to be good electron-transporting (ET) materials and prominent hole blockers (HB) used in organic optoelectronic devices [1,2,3]. 1,3,4-oxadiazoles are the most widely studied class of ET materials due to their electron deficiency, high photoluminescence quantum yield, and good thermal and chemical stabilities [4,5,6,7]. Device performance can be improved by the use of oxadiazoles in the ET layers of both organic light-emitting diodes (OLEDs) and organic solar cells (OSCs), as they help to reduce the operating voltages [8,9,10].
The first reported small organic molecule as the ET layer in OLEDs is 2-tert-butylphenyl-5-biphenyl-1,3,4-oxadiazole (PBD) [11]. Subsequently, the preference of using oxadiazole motifs in polymeric and hyperbranched dendrimeric structures extensively improved the photo- and electroluminescence characteristics for their utilization in green OLED devices [12,13,14,15]. Since oxadiazolediamines with ether links are designed to develop flexible aromatic polyimides [16,17], postpolymer modification of specific carboxylic acid pendant polymers is easily performed by oxadiazole amines via mild reaction conditions, which effectively tunes the dielectric properties of the resulting polymers for specialized applications [18,19]. Recently, a series of 1,3,4-oxadiazoles were tested for their optical nonlinearity. By tuning the electronic properties to study the origin of the nonlinearity in the molecules, various aromatic donors and acceptor functional groups were inserted to oxadiazole frameworks. The literature explicitly indicates that 1,3,4-oxadiazoles have been explored in electroluminescent and light-emitting devices because of their good electron withdrawing effect [20]. With this in mind, chromophores made of oxadiazole moiety were successfully designed and employed in easily processable polymers and nonlinear optical (NLO) responses were studied [21,22].
1,3,4-Oxadiazoles are not only common in materials science but are also used in medicinal chemistry as a potent pharmaceutical scaffold. The improved biological activity and low toxicity of the oxadiazole moiety has attracted a great amount of attention from medicinal chemists for the development of novel drugs [23]. Since heterocyclic azo compounds are well known for their medicinal importance as anti-inflammatory, antibacterial, and antidiabetic compounds [24,25], several alkyl and aryl azo derivatives of 1,3,4-oxadiazoles have been developed and screened for their antimicrobial, anticancer, and in vitro antioxidant properties [24,25].
A number of synthetic routes have been reported for the synthesis of 1,3,4-oxadiazole derivatives [26]. The most commonly used synthetic route includes reactions of acid hydrazides with acid carboxylic acids and direct cyclization of diacylhydrazines using a variety of dehydrating agents such as phosphorous oxychloride, thionyl chloride, phosphorous pentoxide, triflic anhydride, etc. [27]. However, a polyphosphoric-acid-mediated condensation route is a more prominent and convenient path with high purity [28]. Pidugu et al. [29] successfully synthesized the same compound in the form of hydrochloride salt by three steps. In the first step, Boc-glycine was reacted with p-toluic hydrazide in the presence of 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide-hexafluorophosphate (HATU) and N,N-Diisopropylethylamine base (DiPEA). Later, cyclization was done by triphenylphosphine and carbon tetrachloride using trimethylamine base at room temperature. Finally, Boc protection by trifluoroacetic acid (TFA) was cleaved and 1-[5-(4-tolyl)-1,3,4-oxadiazol-2-yl]methanamine HCl salt by hydrochloride ethyl acetate was isolated. However, the protocol described here is the polyphosphoric acid (PPA) condensation route and is more convenient to synthesize 1-[5-(4-tolyl)-1,3,4-oxadiazol-2-yl]methanamine in its free form. The title compound can be exploited easily to other functional clusters or inserted to various functional polymers for pharmacological and technological applications.
2. Results and Discussion
The use of PPA as a reagent for the condensation of 2 and 1 is reported in Scheme 1. The target compound 3 is a grey crystalline powder, is insoluble in water, n-hexane, and has good solubility in tetrahydrofuran, dimethylformamide, methanol, ethanol, isopropanol, and dimethyl sulfoxide. The UV-Vis spectrum of compound 3 was obtained in three solvents—N,N′-dimethylformamide, ethyl alcohol, and tetrahydrofuran—and showed absorption maxima (λmax) at 296, 271, and 279 nm, respectively.
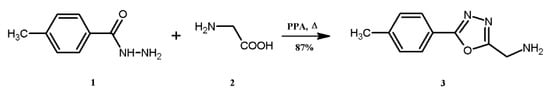
Scheme 1.
Synthesis of 1-[5-(4-tolyl)-1,3,4-oxadiazol-2-yl]methanamine 3.
The 1H and 13C-NMR spectral data of the title compound is in good agreement with the proposed structure. From 1H-NMR spectra (Figure S1), the peaks at δ 8.00 and 7.42 correspond to aromatic protons. The methyl and methylene proton signals appeared at δ 2.39 and 4.05, respectively [30]. 13C-NMR spectra show eight carbon signals (Figure S2). The signals at δ 164.32 and 161.98 correspond to oxadiazole ring carbons. The signal for aromatic carbon attached to the oxadiazole ring and methyl group appeared at δ 121.11 and 142.59, respectively. The aliphatic carbon attached to the oxadiazole moiety appeared at δ 50.24. The signals at δ 130.40 and 127.05 correspond to aromatic carbons of the benzene ring. The chemical shift value at δ 21.59 was ascribed to carbon of methyl functionality [31,32].
3. Experimental Section
3.1. Materials
p-Toluic hydrazide ≥99%, glycine ≥99%, polyphosphoric acid (115% H3PO4 basis), and sodium bicarbonate were procured from Sigma Aldrich, Seoul, Korea. All other chemicals and solvents were of analytical reagent grade and used without any further purification. Double-distilled water was used throughout the study.
3.2. Instrumentaion
NMR spectra were recorded on an Agilent Technologies 400-MR DD2 spectrometer (Agilent, CA, USA). 1H and 13C-NMR spectra were referenced to external SiMe4 (tetramethylsilane) using the residual protio solvent peaks as internal standards (with a 5-mm dual broad-band probe, a 5-mm dual inverse broad-band probe, and a 1.7-mm triple resonance (1H-13C) probe). LCMS measurement was performed on a liquid chromatography system with mass spectrometry detection (Q-TOF)—Agilent ACCURATE MASS 6530 Q TOF LC/MS (Agilent). The elemental analysis was carried out on a VARIO EL III Elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) and the results for C, H, and N were within 0.5% of the theoretical values. FTIR analysis was carried out on a Nicolet 6700 (ATR) (Thermo Fisher Scientific, MA, USA) in the scanning range of 4000–500 cm−1. The resolution was 4 cm−1 with 64 scans and the measurement was done with a germanium crystal. The DSC measurement was performed using the NETZSCH DSC 214 Polyma (Mettler-Toledo GmbH, Giessen, Germany), aluminum crucibles (type Concavus) with pierced lids, and pure nitrogen as the purge gas at a flow rate of 40 mL·min−1. UV-Vis absorption spectra were recorded by a VARIAN-EL08043361, UV-Vis spectrophotometer (Varian, CA, USA) in a scanning range of 200–600 nm at ambient temperature (24–25 °C). UV-Vis spectra of 0.001 M solution of compound 3 in ethanol, tetrahydrofuran, and N,N’-dimethylformamide were recorded with a 1-cm quartz cell in the range of 250–600 nm at ambient temperature.
3.3. Synthesis of 1-[5-(4-Tolyl)-1,3,4-oxadiazol-2-yl]methanamine
Synthesis was performed using oven-dried glassware under an atmosphere of nitrogen. The pulverized mixture of 1 (1.00 g, 6.65 mmol) and 2 (0.499 g, 6.65 mmol) in polyphosphoric acid (20 g) was gradually heated to 120 °C for 2 h with stirring (Scheme 1). Further, the heating was raised to 160 °C for 12 h to accomplish the cyclization reaction, resulting in the formation of the title compound 3. The reaction mixture was cooled to 80 °C and poured slowly on crushed ice (500 g) with agitation. The product was obtained by salting-out the reaction mixture with NaCl (220 g). The obtained grey solids were separated by filtration and suspended in 300 mL of 1 M sodium bicarbonate solution for 1 h and filtered again. The title compound was washed with distilled water until the pH of the filtrate was neutral. The product was vacuum dried at 60 °C for 12 h and recrystallized using ethyl alcohol. The desired product was achieved as a grey microcrystalline powder (1.1 g) in 87%.
Grey microcrystalline powder; m.p. (DSC) onset: 174.63 °C, peak max: 176.20 °C; 1H-NMR (400 MHz, DMSO-d6): δ 8.00 (d, 2H, J = 8.0 Hz), 7.42 (d, 2H, J = 8.0 Hz), 4.45 (s, 2H), 2.39 (s, 3H); 13C-NMR (100 MHz, DMSO-d6): δ 164.32, 161.97, 142.59, 130.40, 127.05, 121.11, 50.24, 21.59; Anal. calcd. for C10H11N3O (189.213): C, 63.48; H, 5.86; N, 22.21. Found: C, 63.29; H, 5.78; N, 21.98; LCMS (ESI): m/z = 189.213 (Actual), 190.066 (Expected for [M + H]+); FTIR (Ge Crystal): 3435, 3375 (N-H Stretch, Primary amine), 3065 (C-H, Aromatic), 1625 (C=N, Oxadiazole), 1520 (C=C, Aromatic), 1315 (CH2), 1190 (C-N), 1025 (C-O-C, Oxadiazole), 755 cm−1 (N-H wag). UV-Vis: λmax 296 (log ε = 3.51), 271 (log ε = 3.52), and 279 (log ε = 3.54) nm in N,N′-Dimethylformamide, ethyl alcohol, and tetrahydrofuran, respectively.
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectra of compound 3, Figure S2: 13C-NMR spectra of compound 3, Figure S3: LCMS spectrum of compound 3, Figure S4: FTIR spectrum of compound 3, Figure S5: DSC spectrum of compound 3, Figure S6: UV-Vis spectrum of 0.001 M solution of compound 3 in (a) N,N′-Dimethylformamide (b) Ethyl alcohol (c) Tetrahydrofuran.
Author Contributions
G.S., designed the synthesis, carryout the experiments for the title compound and wrote the article. E.-J.S., helped in the analysis of the compound. S.-Y.K., supervised the research. All the authors have read and approved the final manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01059649). The work is also supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2018R1A6A1A03025526).
Acknowledgments
We acknowledge Cooperative Equipment Center at KoreaTech for NMR, DSC, UV-vis, FTIR analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, C.; Zhao, P.; Huang, W. New oxadiazole derivatives as promising electron transport materials: Synthesis and characterization of thermal, optical and electrochemical properties. Cent. Eur. J. Chem. 2007, 5, 303–315. [Google Scholar] [CrossRef]
- Kaippamangalath, N.; Gopalakrishnapanicker, U. Synthesis and evaluation of properties of poly(p-phenylenevinylene) based 1,3,4-oxadiazole systems for optoelectronics and nonlinear optical applications. Polym. Int. 2016, 65, 1221–1231. [Google Scholar] [CrossRef]
- Chandrakantha, B.; Isloor, A.M.; Philip, R.; Mohesh, M.; Shetty, P.; Vijesh, A.M. Synthesis and nonlinear optical characterization of new 1,3,4-oxadiazoles. Bull. Mater. Sci. 2011, 34, 887–891. [Google Scholar] [CrossRef]
- Ichikawa, M.; Kawaguchi, T.; Kobayashi, K.; Miki, T.; Furukawa, K.; Koyama, T.; Taniguchi, Y. Bipyridyl oxadiazoles as efficient and durable electron-transporting and hole-blocking molecular materials. J. Mater. Chem. 2006, 16, 221–225. [Google Scholar] [CrossRef]
- Carli, S.; Baena, J.P.C.; Marianetti, G.; Marchetti, N.; Lessi, M.; Abate, A.; Caramori, S.; Grätzel, M.; Bellina, F.; Bignozzi, C.A.; et al. A New 1,3,4-Oxadiazole-Based Hole-Transport Material for Efficient CH3NH3PbBr3 Perovskite Solar Cells. ChemSusChem 2016, 9, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chidirala, S.; Ulla, H.; Valaboju, A.; Kiran, M.R.; Mohanty, M.E.; Satyanarayan, M.N.; Umesh, G.; Bhanuprakash, K.; Rao, V.J. Pyrene–Oxadiazoles for Organic Light-Emitting Diodes: Triplet to Singlet Energy Transfer and Role of Hole-Injection/Hole-Blocking Materials. J. Org. Chem. 2016, 81, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Suwiński, J.; Szczepankiewicz, W. Five-membered Rings—Triazoles, Oxadiazoles, Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elseiver: Oxford, UK, 2008; Volume 5, Chapter 5.06; pp. 397–466. ISBN 9780080449920. [Google Scholar]
- Kim, J.H.; Lee, H. Enhancement of efficiency in luminescent polymer by incorporation of conjugated 1,3,4-oxadiazole side chains as hole-blocker/electron-transporter. Synth. Met. 2004, 143, 13–19. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Bi, Z.; Yu, T.; Ma, W.; Feng, K.; Li, Y.; Peng, Q. Highly Efficient Nonfullerene Polymer Solar Cells Enabled by a Copper(I) Coordination Strategy Employing an 1,3,4-Oxadiazole-Containing Wide-Bandgap Copolymer Donor. Adv. Mater. 2018, 30, 1800737. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, U.; Hussein, I.A.; Daud, M. New 1,3,4-Oxadiazole Based Photosensitizers for Dye Sensitized Solar Cells (DSSCs). Int. J. Photoenergy 2015, 2015. [Google Scholar] [CrossRef]
- Lü, Z.; Deng, Z.; Zheng, J.; Xu, D.; Chen, Z.; Zhou, E.; Wang, Y. Organic light-emitting diodes with 2-(4-biphenylyl)-5(4-tert-butyl-phenyl)-1,3,4-oxadiazole layer inserted between hole-injecting and hole-transporting layers. Vacuum 2010, 84, 1287–1290. [Google Scholar] [CrossRef]
- Ma, D.; Lupton, J.M.; Samuel, D.W.; Lo, S.C.; Burn, P.L. Bright electroluminescence from a conjugated dendrimer. Appl. Phys. Lett. 2002, 81, 2285–2287. [Google Scholar] [CrossRef]
- Paun, A.; Hadade, N.D.; Paraschivescu, C.C.; Matache, M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. [Google Scholar] [CrossRef]
- Kim, S.J.; Zhang, Y.; Zuniga, C.; Barlow, S.; Marder, S.R.; Kippelen, B. Efficient green OLED devices with an emissive layer comprised of phosphor-doped carbazole/bis-oxadiazole side-chain polymer blends. Org. Electron. 2011, 12, 492–496. [Google Scholar] [CrossRef]
- Ichikava, M.; Shimizu, C.; Koyama, T.; Taniguchi, Y. Improvement of photovoltaic performances of organic thin-film solar cells by fast electron mobility oxadiazole as an exciton blocking layer material. Phys. Stat. Sol. 2008, 205, 1222–1225. [Google Scholar] [CrossRef]
- Mansoori, Y.; Ghanbari, M. Novel polyimides obtained from a new aromatic diamine (BAPO) containing pyridine and 1,3,4-oxadiazole moieties for removal of Co(II) and Ni(II) ions. Polym. Adv. Technol. 2015, 26, 658–664. [Google Scholar] [CrossRef]
- Mercer, F.W.; McKenzie, M.T. Dielectric and thermal characterization of fluorinated polyimides containing heterocyclic moieties. High Perform. Polym. 1993, 5, 97–106. [Google Scholar] [CrossRef]
- Ganesh, S.D.; Pai, V.K.; Kariduraganavar, M.Y.; Jayanna, M.B. Functional Aromatic Poly(1,3,4-Oxadiazole-Ether)s with Benzimidazole Pendants: Synthesis, Thermal and Dielectric Studies. Int. Sch. Res. Not. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.D.; Pai, V.K.; Kariduraganavar, M.Y.; Jayanna, M.B. Thermal and Dielectric Behavior Studies of Poly(Arylene Ether Sulfone)s with Sulfonated and Phosphonated Pendants. J. Mater. 2016, 2016. [Google Scholar] [CrossRef]
- Bruma, M.; Hamciuc, E.; Schulz, B.; Kopnick, T.; Kaminorz, Y.; Robison, J. Synthesis and study of new polyamides with side oxadiazole rings. J. Appl. Polym. Sci. 2003, 87, 714–721. [Google Scholar] [CrossRef]
- Tasaganva, R.G.; Tambe, S.M.; Kariduraganavar, M.Y. Synthesis and characterization of thermally stable second-order nonlinear optical side-chain polyurethanes containing nitro-substituted oxadiazole and thiazole chromophores. J. Mol. Struct. 2011, 1000, 10–23. [Google Scholar] [CrossRef]
- Tasaganva, R.G.; Doddamani, R.V.; Inamdar, S.R.; Kariduraganavar, M.Y. Synthesis of thermally stable new polyurethanes containing nitro-substituted 1,3,4-oxadiazole chromophores for second order nonlinear optical applications. Optik 2015, 126, 4991–5000. [Google Scholar] [CrossRef]
- Sun, J.; Makawana, J.A.; Zhu, H.L. 1,3,4-Oxadiazole Derivatives as Potential Biological Agents. Mini-Rev. Med. Chem. 2013, 13, 1725–1743. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, A.H.; Keshavayya, J.; Peethambar, S.K.; Hoskeri, H.J. Synthesis and biological activities of Bis alkyl 1,3,4-oxadiazole incorporated azo dye derivatives. Arabian J. Chem. 2016, 9, S1643–S1648. [Google Scholar] [CrossRef]
- Kadi, A.A.; El-Brollosy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.W. Product Class 8: 1,3,4-Oxadiazoles. In Science of Synthesis; Storr, R.C., Gilchrist, T.L., Eds.; Georg Thieme: Stuttgart, Germany, 2003; Volume 13, Chapter 13.8; pp. 219–252. [Google Scholar]
- Mickevičius, V.; Vaickelioniene, R.; Sapijanskaite, B. Synthesis of substituted 1,3,4-oxadiazole derivatives. Chem. Heterocycl. Compd. 2009, 45, 215–218. [Google Scholar] [CrossRef]
- Gomes, D.; Borges, C.P.; Pinto, J.C. Study of the synthesis of poly(4,4′-diphenylether-1,3,4-oxadiazole) in solutions of poly(phosphoric acid). Polymer 2001, 42, 851–865. [Google Scholar] [CrossRef]
- Pidugu, V.R.; Yarla, N.S.; Pedada, S.R.; Kalle, A.M.; Satya, A.K. Design and synthesis of novel HDAC8 inhibitory 2,5-disubstituted-1,3,4-oxadiazoles containing glycine and alanine hybrids with anticancer activity. Bioorg. Med. Chem. 2016, 24, 5611–5617. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyi, I.S.; Belen’kii, L.I.; Struchkova, M.I.; Krayushkin, M.M. 1H and 13C NMR spectra of 1,5-disubstituted 1,3,4-oxadiazoles. Chem. Heterocycl. Compd. 1994, 30, 729–737. [Google Scholar] [CrossRef]
- Desai, N.C.; Dodiya, A.M.; Rajpara, K.M.; Rupala, Y.M. Synthesis and antimicrobial screening of 1,3,4-oxadiazole and clubbed thiophene derivatives. J. Saudi Chem. Soc. 2014, 18, 255–261. [Google Scholar] [CrossRef]
- Kim, B.S.; Ahn, S.; Koh, D.; Cho, S.M.; Song, Y.W.; Sung, J.; Lim, Y. 1H and 13C NMR characterization of 1,3,4-oxadiazole derivatives. Magn. Reson. Chem. 2018, 56, 782–791. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).