(E)-3-[4-(Pent-4-en-1-yloxy)phenyl]acrylicc Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Initial Screening of the Reaction Conditions Using a Batch Reactor
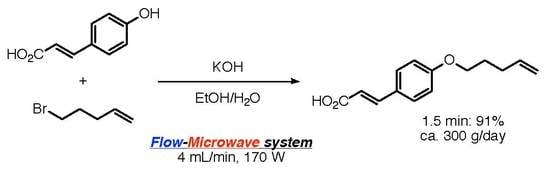
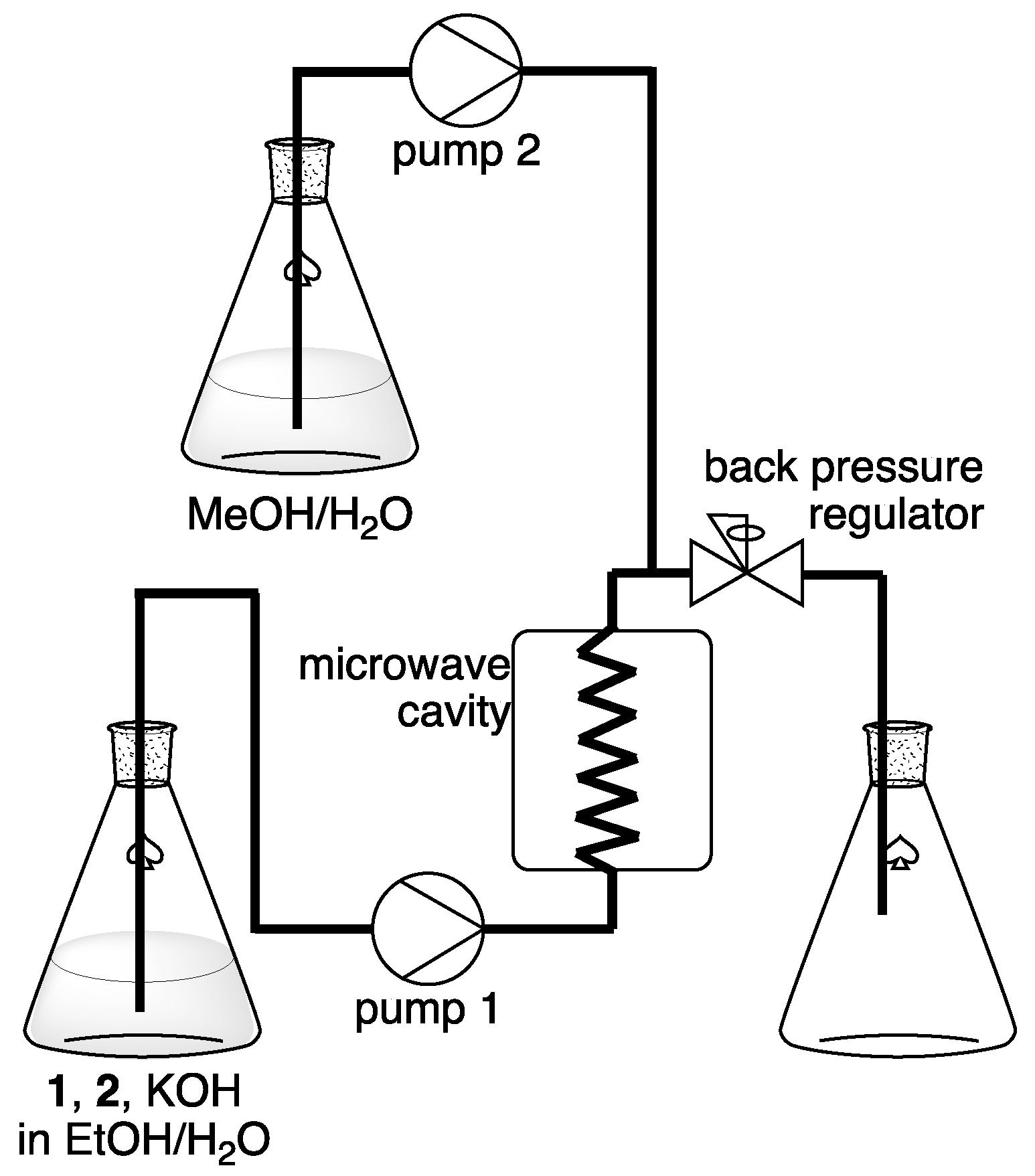
2.2. Continuous Williamson Ether Synthesis Using our Flow-Microwave Applicator
3. Experiments
3.1. General
3.2. Typical Procedure of Willimason Ether Synthesis Using Flow-Microwave System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harada, Y.; Sakajiri, K.; Kuwahara, H.; Kang, S.; Watanabe, J.; Tokita, M. Cholesteric films exhibiting expanded or split reflection bands prepared by atmospheric photopolymerisation of diacrylic nematic monomer doped with a photoresponsive chiral dopant. J. Mater. Chem. C 2015, 3, 3790–3794. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.; Ghosh, S.K.; Sar, P.; Ghosh, A.; Saha, R.; Saha, B. A reveiw on the advancement of ether synthesis from organic solvent to water. RSC Adv. 2016, 6, 69605–69614. [Google Scholar] [CrossRef]
- Fuhrmann, E.; Talbiersky, J. Synthesis of Alkyl Aryl Ethers by Catalytic Williamson Ether Synthesis with Weak Alkylation Agents. Org. Proc. Res. Dev. 2005, 9, 206–211. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; WILEY-VHC: Weinheim, Germany, 2005. [Google Scholar]
- Horikoshi, S.; Serpone, N. Microwaves in Catalysis; WILEY-VHC: Weinheim, Germany, 2016. [Google Scholar]
- Paul, S.; Gupta, M. Zinc-catalyzed Williamson ether synthesis in the absence of base. Tetrahedron Lett. 2004, 45, 8825–8829. [Google Scholar] [CrossRef]
- Reddy, K.R.; Rajanna, K.C.; Ramgopal, S.; Kumar, M.S.; Sana, S. Environmentally Benign Synthetic Protocol for O-Alkylation of β-Naphthols and Hydroxy Pyridines in Aqueous Micellar Media. Green Sustain. Chem. 2012, 2, 123–132. [Google Scholar] [CrossRef][Green Version]
- Baar, M.R.; Gammerdinger, W.; Leap, J.; Morales, E.; Shikora, J.; Weber, M.H. Pedagogical Comparison of Five Reactions Performed under Microwave Heating in Multi-Mode versus Mono-Mode Ovens: Diels–Alder Cycloaddition, Wittig Salt Formation, E2 Dehydrohalogenation To Form an Alkyne, Williamson Ether Synthesis, and Fischer Esterification. J. Chem. Educ. 2014, 91, 1720–1724. [Google Scholar]
- Otero, E.; Vergara, S.; Robledo, S.M.; Cardona, W.; Carda, M.; Vélez, I.D.; Rojas, C.; Otálvaro, F. Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids. Molecules 2014, 19, 13251–13266. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Flow “Fine” Synthesis: High Yielding and Selective Organic Synthesis by Flow Methods. Chem. Asian J. 2016, 11, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology–A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef]
- Cantillo, D.; Kappe, C.O. Halogenation of organic compounds using continuous flow and microreactor technology. React. Chem. Eng. 2017, 2, 7–19. [Google Scholar] [CrossRef]
- Shukla, C.A.; Kulkarni, A.A. Automating multistep flow synthesis: Approach and challenges in integrating chemistry, machines and logic. Beilstein J. Org. Chem. 2017, 13, 960–987. [Google Scholar] [CrossRef] [PubMed]
- Bergamelli, F.; Iannelli, M.; Marafie, J.A.; Moseley, J.D. A Commercial Continuous Flow Microwave Reactor Evaluated for Scale-Up. Org. Proc. Res. Dev. 2010, 14, 926–930. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. The Microwave-to-Flow Paradigm: Translating High-Temperature Batch Microwave Chemistry to Scalable Continuous-Flow Process. Chem. Eur. J. 2011, 17, 11956–11968. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-T.; Chiou, S.-H.; Wang, K.-T. Preparative Scale Organic Synthesis using a Kitchen Microwave Oven. J. Chem. Soc. Chem. Commun. 1990, 807–809. [Google Scholar] [CrossRef]
- Cablewski, T.; Faux, A.F.; Strauss, C.R. Development and Application of a Continuous Microwave Reactor for Organic Synthesis. J. Org. Chem. 1994, 59, 3408–3412. [Google Scholar] [CrossRef]
- Yokozawa, S.; Ohneda, N.; Muramatsu, K.; Okamoto, T.; Odajima, H.; Ikawa, T.; Sugiyama, J.; Fujita, M.; Sawairi, T.; Egami, H.; et al. Development of a highly efficient single-mode microwave applicator with a resonant cavity and its application to continuous flow syntheses. RSC Adv. 2015, 5, 10204–10210. [Google Scholar] [CrossRef]
- Ichikawa, T.; Mizuno, M.; Ueda, S.; Ohneda, N.; Odajima, H.; Sawama, Y.; Monguchi, Y.; Sajiki, H. A practical method for heterogeneously-catalyzed Mizoroki-Heck reaction: Flow system with adjustment of microwave resonance as an energy source. Tetrahedron Lett. 2018, 74, 1810–1816. [Google Scholar] [CrossRef]
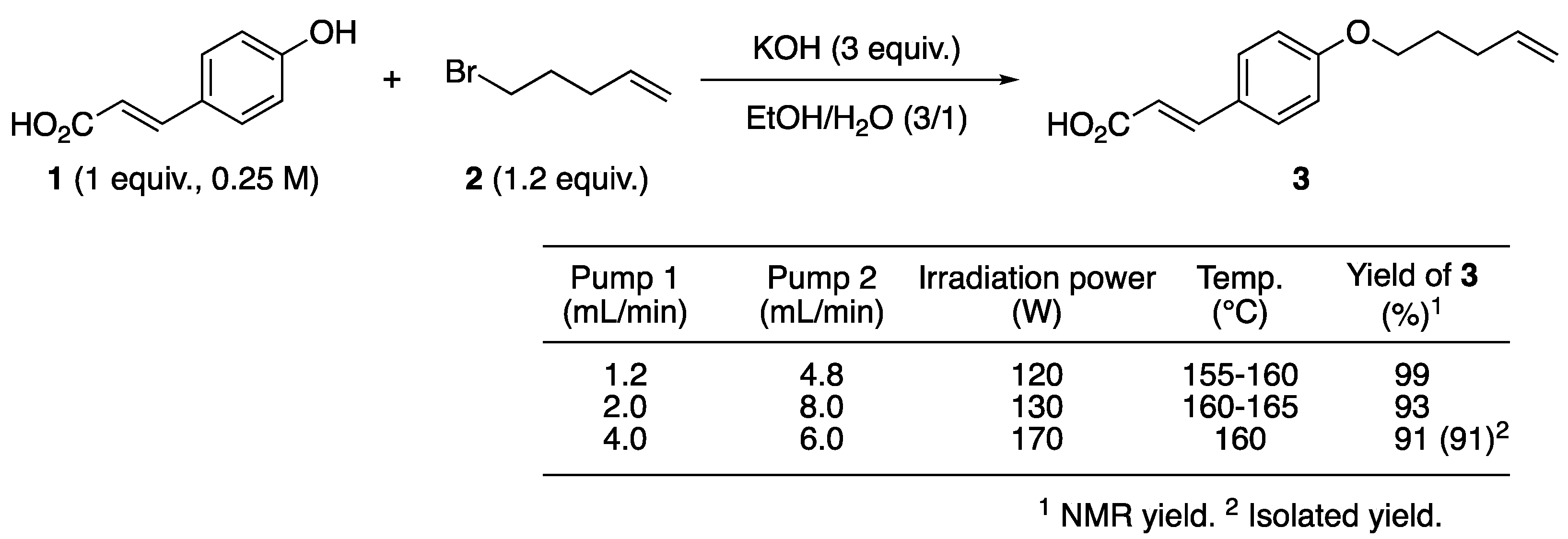
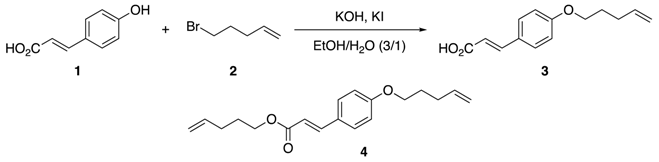
| Entry | Heat Source | Time | KOH (equiv) | KI (equiv) | Temp. (°C) | Yield of 3 (%) 2 |
|---|---|---|---|---|---|---|
| 1 | oil bath | 24 h | 2.0 | 0.5 | 90 | 96 |
| 2 | oil bath | 10 min | 2.0 | 0.5 | 90 | 23 |
| 3 | microwave | 10 min | 2.0 | 0.5 | 90 | 32 |
| 4 | microwave | 10 min | 2.5 | 0 | 150 | 78 3 |
| 5 4 | microwave | 10 min | 3.0 | 0 | 150 | 91 |
| 6 4,5 | microwave | 10 min | 3.0 | 0 | 150 | 86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egami, H.; Sawairi, T.; Tamaoki, S.; Ohneda, N.; Okamoto, T.; Odajima, H.; Hamashima, Y. (E)-3-[4-(Pent-4-en-1-yloxy)phenyl]acrylicc Acid. Molbank 2018, 2018, M996. https://doi.org/10.3390/M996
Egami H, Sawairi T, Tamaoki S, Ohneda N, Okamoto T, Odajima H, Hamashima Y. (E)-3-[4-(Pent-4-en-1-yloxy)phenyl]acrylicc Acid. Molbank. 2018; 2018(2):M996. https://doi.org/10.3390/M996
Chicago/Turabian StyleEgami, Hiromichi, Taira Sawairi, Souma Tamaoki, Noriyuki Ohneda, Tadashi Okamoto, Hiromichi Odajima, and Yoshitaka Hamashima. 2018. "(E)-3-[4-(Pent-4-en-1-yloxy)phenyl]acrylicc Acid" Molbank 2018, no. 2: M996. https://doi.org/10.3390/M996
APA StyleEgami, H., Sawairi, T., Tamaoki, S., Ohneda, N., Okamoto, T., Odajima, H., & Hamashima, Y. (2018). (E)-3-[4-(Pent-4-en-1-yloxy)phenyl]acrylicc Acid. Molbank, 2018(2), M996. https://doi.org/10.3390/M996