Abstract
The compound 2,4-bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol was synthesized with a yield of approximately 80% by the reaction of 2,4-bis(2,6-diisopropylphenylimino)pentan-3-one with trimethylaluminum, which was followed by hydrolysis with an aqueous NaOH solution. A chemoselective addition to the C=O bond occurred in this reaction. The structure was confirmed by X-ray crystallography. This new compound was also fully characterized by 1H, 13C-NMR spectroscopy and 1H-13C HSQC spectroscopy, mass spectrometry and elemental analysis.
1. Introduction
β-Diketiminates (Scheme 1, I) or β-diimines (the tautomers of β-diketiminates, Scheme 1, II) are well known as the spectator ligands to stabilize metallic complexes. The steric and electronic properties of β-diketiminates or β-diimines can be easily modified with the appropriate substituents. Thus, these ligands have been widely applied in the metal-catalyzed organic reactions and polymerizations [1,2,3,4]. However, the synthesis of β-diimines with a functional group at the α-site remains scarce (Scheme 1, III–V) [5,6,7,8]. One notable example is that the introduction of the carbonyl functionality at the α-site (α-keto-β-diimine, Scheme 1, V) enables an improvement in the control of nickel-catalyzed polymerization [8]. Furthermore, the reaction of the α-keto-β-diimine nickel complex with trimethylaluminum (TMA) results in the formation of binuclear organometallic species (Scheme 1, VI) [9]. On the basis of these works, we decided to synthesize the α-hydroxyl-β-diimine by the reduction of the C=O double bond of α-keto-β-diimine. Since the reduction of C=N double bonds may also occur in this reaction, selective reduction of only the C=O double bond is the main difficulty that we had to consider [10]. In this contribution, the first available preparation and characterization of 2,4-bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol were described.
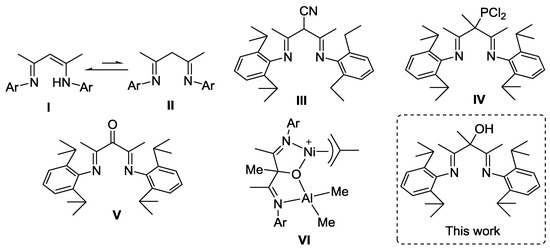
Scheme 1.
Reported β-diimines with the functional group at the α-site and our current work.
2. Results and Discussion
Previously, the selective reductive addition of the α-imino ketones with the organoaluminium regents has been reported [11,12,13]. As a result, trimethylaluminum (TMA) was chosen as a reducing agent to obtain the desired product. As shown in Scheme 2, treating 2,4-bis(2,6-diisopropylphenylimino)pentan-3-one with 1.3 or 3 equiv of AlMe3 at the reflux temperature for 10 h created the aluminum complex in situ, before the reaction mixture was carefully hydrolyzed with an aqueous NaOH solution. According to the reported complex VI (Scheme 1) [8], the intermediate aluminum salt is deemed to be N,O-bound with a tetrahedral arrangement (Scheme 2). The desired product was obtained in high yields of up to 79.3%. To our surprise, the selective reduction of C=O double bonds still occurs even in the presence of an excess proportion of AlMe3. This was the first reported compound, which was fully characterized by 1H, 13C-NMR spectroscopy and 1H-13C HSQC spectroscopy, mass spectrometry (Figures S1–S4), elemental analysis and X-ray crystallography (Table S1). In CDCl3, the characteristic peak in the 1H-NMR for the products is at approximately δ = 6.27 ppm, which corresponds to the proton of the hydroxyl group. The chemical shift at 80.63 ppm in the 13C-NMR represents the signals for the C-OH of the compound. The chemical shifts of other protons and carbons were further assigned by 1H, 13C-NMR spectroscopy and the 1H-13C HSQC spectroscopy (see Materials and Methods).
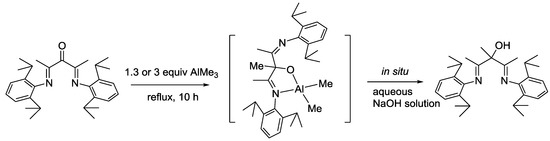
Scheme 2.
Synthesis of 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol.
The recrystallization of this compound in the ethanol solution at −20 °C created single crystals that were suitable for X-ray diffraction. The molecular structure of the product is shown in Figure 1. It is clear that the tertiary carbon is located on the hydroxyl group rather than N(1) or/and N(2), which further confirms that the selective reductive addition is carried out at the C=O double bonds. No intermolecular interactions were observed in this structure.
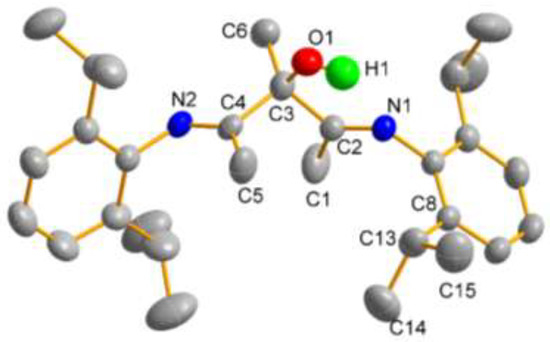
Figure 1.
Structure of 2,4-bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol obtained by X-ray crystallography. Thermal ellipsoids are shown at the probability level of 30%. Hydrogen atoms, except O-H, have been omitted for clarity. The hydroxyl group and methyl group on C3 are disordered over two sites, while the isopropyl group attached to C8 is also disordered over two sites. Only one orientation is shown here.
3. Materials and Methods
3.1. General Information
The compound 2,4-bis(2,6-diisopropylphenylimino)pentan-3-one was synthesized according to the previously reported method [7]. All other reagents were purchased from commercial sources and used without purification. The 1H-NMR spectra were captured in 5-mm NMR tubes at 298 K on Bruker DPX 500 (1H = 500.13 MHz) spectrometers (Bruker, Karlsruhe, Germany) using TMS as an internal standard and CDCl3 as the solvent. The 13C-NMR spectra were referenced to the residual solvent (CHCl3, 77.16 ppm) for chloroform-d1. The elemental analysis was performed by the Analytical Center of the University of Science and Technology of China. A mass spectrum of the compound was recorded on a Thermo LTQ Orbitrap XL (ESI+, Thermo Fisher Scientific, Waltham, MA, USA). The X-ray diffraction data were collected at 298(2) K on a Bruker Smart CCD area detector (Bruker, Karlsruhe, Germany) with graphite-monochromated MoKα radiation (λ = 0.71073 Å). The structures were solved by direct methods, before further refinement with full-matrix least-squares on F2 was obtained with the SHELXL program package [14,15].
3.2. Synthesis of 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol
Under a nitrogen atmosphere, trimethylaluminum (TMA) (1.3 equiv, 6.0 mmol, 3.8 mL, 1.6 M in toluene) was injected slowly through a syringe into a solution of 2,4-bis(2,6-diisopropylphenylimino)pentan-3-one (2.0 g, 4.6 mmol) in 45 mL of toluene at room temperature. The reaction was heated to reflux for 10 h. After the solution was cooled to the ambient temperature, the reaction mixture was carefully hydrolyzed with 5% aqueous NaOH solution in an ice bath. After extraction with ethyl acetate, the organic layer was dried with anhydrous MgSO4 and filtered. The solvent was removed under reduced pressure. The crude product was isolated as a viscous oil. After recrystallization from ethanol at −20 °C, the purified white crystals can be obtained with a yield of 78.7% (1.62 g). When 3 equiv of TMA was employed, the same product was obtained with a yield of 79.3% (1.64 g). The 1H-NMR (400 MHz, CDCl3) obtained δ 7.18–7.05 (m, 6H, aryl-H), 6.27 (s, 1H, OH), 2.73 (heptet, J = 6.8 Hz, 2H, CH(CH3)2), 2.66 (heptet, J = 6.8 Hz, 2H, CH(CH3)2), 1.85 (s, 6H, N=C(CH3)), 1.82 (s, 3H, CH3-C-OH), 1.16 (two overlapping doublets, 24H, CH(CH3)2). The 13C-NMR (126 MHz, CDCl3) obtained δ 172.93 (CH3-C=N), 144.94 (N-C), 136.38 (aryl-C), 136.16 (aryl-C), 124.14 (aryl-C), 123.22 (aryl-C), 123.19 (aryl-C) 80.63 (CH3-C-OH), 28.20 (CH(CH3)2), 24.56 (CH3-C-OH), 23.53 (CH(CH3)2), 23.43 (CH(CH3)2), 23.08 (CH(CH3)2), 22.83 (CH(CH3)2), 16.01 (CH3-C=N). ESI-MS (m/z): calcd. for C30H45N2O: 449.3532, found: 449.3521 [M + H]+. Anal. Calcd. for C30H44N2O: C, 80.31; H, 9.88; N, 6.24; Found: C, 80.25; H, 9.89; N, 6.27.
The Crystal Data for C30H44N2O (M = 448.67 g/mol) are: triclinic, space group P-1, a = 8.9713(8) Å, b = 13.5010(11) Å, c = 13.5357(12) Å, α = 109.324(3) °, β = 99.633(2)°, γ = 104.420(2)°, V =1441.3(2) ų, Z = 2, T = 298(2) K, μ(MoKα) = 0.062 mm−1, Dcalc = 1.034 g/cm3, 7285 reflections measured (−8 ≤ h ≤ 10, −16 ≤ h ≤ 16, −16 ≤ 1 ≤ 15) and 5007 unique (Rint = 0.0281), which were used in all calculations. The final R1 was 0.0839 (I > 2σ(I)) and the wR2 was 0.2452 (all data). The molecule is disordered, the hydroxyl group and methyl group on C3 are disordered over the two sites with occupancies of 0.51 (2): 0.49(2), while the isopropyl group attached to C8 is disordered over the two sites with occupancies of 0.60 (4): 0.40 (4).
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol in CDCl3, Figure S2: 13C-NMR spectrum of 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol in CDCl3, Figure S3: 1H-13C HSQC spectrum of 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol in CDCl3, Figure S4: ESI-MS of 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol, Table S1: Crystal data and structure refinement for 2,4-Bis[(2,6-diisopropylphenyl)imino]-3-methylpentan-3-ol, a CIF file and a CheckCIF report for the title compound. CCDC 1828820 also contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Author Contributions
L.G. and W.S. conceived and designed the experiments; W.K. and W.S. performed the experiments; W.K. and Q.D. analyzed the data; L.G. and Z.L. wrote the paper.
Funding
This research was funded by [the National Natural Science Foundation of China] grant number [21671118] and [Shandong Provincial Natural Science Foundation] grant number [ZR2018MB023].
Acknowledgments
We thank the Key Laboratory of Polymeric Composite & Functional Materials of Ministry of Education (PCFM-2017-01), the Taishan Scholars Program, Foundation of Qufu Normal University (xkJ201603) and College Students Innovation Project for support.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Bourget-Merle, L.; Lappert, M.F.; Severn, J.R. The chemistry of β-diketiminatometal complexes. Chem. Rev. 2002, 102, 3031–3065. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.; Mclain, S.J.; Parthasarathy, A.; Marshall, W.J.; Calabrese, J.C.; Arthur, S.D. Electrophilic metal precursors and a β-diimine ligand for nickel(II)- and palladium(II)-catalyzed ethylene polymerization. Organometallics 1997, 16, 1514–1516. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2015, 6, 428–441. [Google Scholar] [CrossRef]
- Guo, L.H.; Liu, W.J.; Chen, C.L. Late transition metal catalyzed a-olefin polymerization and copolymerization with polar monomers. Mater. Chem. Front. 2017, 1, 2487–2494. [Google Scholar] [CrossRef]
- Burford, N.; D’eon, M.; Ragogna, P.J.; McDonald, R.; Ferguson, M.J. Synthesis and structures of complexes demonstrating the coordinative versatility of the 2,4-diimino-3-phosphinopentene anion (γ-phosphino-ß-diketiminate). Inorg. Chem. 2004, 43, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.D.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. High-activity, single-site catalysts for the alternating copolymerization of CO2 and propylene oxide. J. Am. Chem. Soc. 2002, 124, 14284–14285. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, D.; Lu, Z.; Reeske, G.; Moore, J.A.; Cowley, A.H. An N,N′-chelated phosphenium cation supported by a β-diketiminate ligand. Chem. Commun. 2006, 3501–3503. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, J.D.; Rojas, R.S.; Serrano, A.V.; Ohtaki, H.; Galland, G.B.; Wu, G.; Bazan, G.C. Nickel α-keto-β-diimine initiators for olefin polymerization. Angew. Chem. Int. Ed. 2009, 48, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, J.D.; Koretz, Z.A.; Wu, G.; Bazan, G.C. Well-defined cationic methallyl α-keto-β-diimine. complexes of nickel. Angew. Chem. Int. Ed. 2010, 49, 7890–7894. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.T.; Mair, F.S.; Pritchard, R.G.; Warren, J.E.; Woods, R.J. Borane and alane reductions of bulky N,N′-diaryl-1,3-diimines: Structural characterization of products and intermediates in the diastereoselective synthesis of 1,3-diamines. Dalton Trans. 2003, 3792–3798. [Google Scholar] [CrossRef]
- Van Vilet, M.R.P.; van Koten, G.; Rotteveel, M.A.; Schrap, M.; Vrieze, K. Reactivity of l-Aza-4-oxo-l, 3-butadienes (α-imino ketones) toward triorganoaluminum reagents. 1. X-ray crystal structures of [(AlMe3)2{(σ,σ-N,O-(MeN=C(Ph)C(Ph)=O)}], with a N,O-bridge-bonded α-imino ketone, and of Me2Al(t-Bu)N = CHC(Me),OAIMe3, a carbonyl alkylated product which is o-coordinated to AIMe3. Organometallics 1986, 5, 1389–1394. [Google Scholar]
- Van Vilet, M.R.P.; van Koten, G.; de Keijser, M.S.; Vrieze, K. Reactivity of 1-Aza-4-oxa-1,3-butadienes (α-imino ketones) toward triorganoalumlnum reagents. 2. syntheses of triorganoaluminum α-imho ketone coordination complexes, diorganoaluminum (α-imino) alkoxides, and diorganoaluminum (α-imino)enolates and study of their dynamic behavior insolution (1H and 13C-NMR). Organometallics 1987, 6, 1652–1664. [Google Scholar]
- Tang, X.; Huang, Y.T.; Liu, H.; Liu, R.Z.; Shen, D.S.; Liu, N.; Liu, F.S. α-Hydroxyimine palladium complexes: Synthesis, molecular structure, and their activities towards the suzukie-miyaura cross-coupling reaction. J. Organomet. Chem. 2013, 729, 95–102. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mcardle, P. Oscail, a program package for small-molecule single-crystal crystallography with crystal morphology prediction and molecular modelling. J. Appl. Crystallogr. 2017, 50, 320–326. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).