Abstract
Cyclo-condensation of N-methyl-2-bromoaniline with chlorocarbonylsulfenyl chloride (CCSC) promoted by PhNMe2 and AlCl3, afforded N-methyl-2-bromo-2(3H)-benzothiazol-2-one in good yield. Miyaura–Ishiyama cross-coupling of this brominated 2(3H)-benzothiazol-2-one with bis(pinacolato)diborone [(pin)2B2] produced a novel N-methyl-4-(pin)B-2(3H)-benzothiazol-2-one (3) using (pin)2B2 in the presence of the PdCl2(PPh3)2 catalyst. The obtained 4-(pin)B compound is regarded as a new entry for the library of Suzuki–Miyaura cross-coupling reactions.
1. Introduction
N-Substituted 2(3H)-benzothiazol-2-ones (1) are well-investigated S,N-containing heterocycles that are incorporated into various pharmaceuticals and agrochemicals [1] (Figure 1). Representative studies of 1 include the following: (1) tiaramide as a characteristic and useful anti-allergic drug [2]; (2) benazoline as a useful selective herbicide [3]; (3) chlobenthiazone as a potent agrochemical fungicide [4,5]; and (4) natural mevashuntin as a unique metabolite of an hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitor [6] and as an efficient target for total synthesis [7]. Other notable pharmaceuticals have also been reported [8,9,10,11].
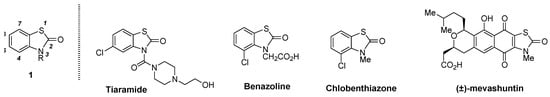
Figure 1.
Representative biologically active compounds containing the N-substituted 2(3H)-benzothiazol-2-one structure.
Compared with simple unsubstituted, 6-chlorinated, and 5-acyl (or alkyl)-substituted N-alkyl-2(3H)-benzothiazol-2-ones, the synthesis of more inaccessible 4-substituted analogues is quite limited due to the three stereocongested contiguous substituents on the 4,8,9-positions. To the best of our knowledge, only three compounds containing the 4-substituted N-alkyl-2(3H)-benzothiazol-2-one structure have been reported: benazoline [3], chlobenthiazone [4,5], and mevashuntin [6] (Figure 1).
Among several synthetic approaches, one of the most straightforward forms of synthesis of N-alkyl-2(3H)-benzothiazol-2-ones utilizes cyclo-condensation of N-alkylaniline with chlorocarbonylsulfenyl chloride (ClC=OSCl, abbreviated CCSC) (2) [12], a unique commercially available bifunctional electrophilic reagent (Figure 2). The preparation of 2 on a >100 g scale was disclosed in the patent by the Bayer group [12]. Zumack and Kühle addressed the notable chemistry of CCSC (2) in their impressive review [13]; 2 serves as a key building blocks for various S,N-containing heterocyclic compounds. In connection with our studies utilizing 2 for the synthesis of S,N-containing heterocycles with a -COS- linkage, we reported on the synthesis of: (1) N-alkyl-2(3H)-benzothiazol-2-ones from N-alkylanilines [14]; (2) N-chloromethyl-2(3H)-benzothiazol-2-ones from N-aryltriazines [15]; (3) three S,N-heterocyclic compounds utilizing α-methoxycarbonylsulfenylation of ketones and aldehydes [16]; and (4) 1,3,4-(3H,6H)-thiadiazin-2-ones and 3(2H)-(N,N-dimethylamino)thiazolones from hydrazones [17].
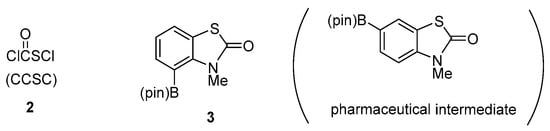
Figure 2.
Chlorocarbonylsulfenyl chloride (CCSC) (2) and 4-N-methyl-4-(pin)B-2(3H)-benzothiazol-2-one derivative 3.
Our recent interest in cross-coupling reactions, directed towards medicinal and process chemistry, [18,19,20,21,22], led us to investigate a concise synthesis of novel 4-(pinacolato)borane (pin)B derivative 3 derived from N-methyl-4-bromo-2(3H)-benzothiazol-2-one (5), which could serve as a convenient substrate for Suzuki–Miyaura cross-coupling reactions (Figure 2). A literature survey using SciFinder® revealed that a 6-(pin)B analogue was reported as the synthetic intermediate for: (1) inhibitors of matrix metalloproteinases (MMPs) and the production of tumor necrosis factor α (TNF α) [23]; (2) treatment of inflammatory respiratory diseases [24]; (3) modulators of aldosterone synthase and/or 11-β hydroxylase [25]; and (4) inhibitors of IKKβ (IκB Kinase-β) kinase [26]. Taking this background into account, we planned the synthesis of the less accessible and novel 4-(pinB) regioisomer 3.
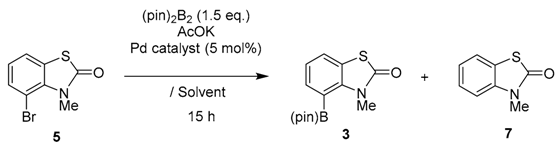
Scheme 1 shows the synthetic route for target compound 3. Monomethylation of 2-bromoaniline gave N-methyl-2-bromoaniline (4) in 90% yield using the method of Barluenga and coworkers [27]. Cyclo-condensation of 4 using CCSC (2)/PhNMe2-combined reagents, followed by the treatment with AlCl3, afforded N-methyl-4-bromo-2(3H)-benzothiazol-2-one (5) in 54% yield. To prepare the boronic acid derivative we initially examined the lithiation of 5 using n-BuLi or t-BuMgCl at −78 °C, followed by treatment with B(OMe)3. The reaction was sluggish, however, and only gave trace amounts of boronic acid derivative 6. Thus, we turned our attention to investigating the more neutral Miyaura–Ishiyama protocol using bis(pinacolato)diborone [(pin)2B2] [28] to obtain 4-(pinacolato)borane [(pin)B] derivative 3.
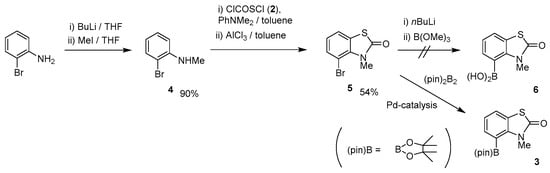
Scheme 1.
Synthetic route for (pin)B derivative 3.
As expected, compared with the preparation of 6-(pin)B isomer, the reaction of 5 gave poor results under the identical conditions [23] due to higher stereocongestion; a considerable reduction to form byproduct 7 was observed. To solve the problem, various Pd-catalysis conditions were screened and these results are listed in Table 1. The most standard method using a PdCl2(dppf) catalyst under several conditions resulted in the formation of 3 in a maximum 30% yield with 7 (7–78%) as main product (entries 1–6). Pd catalysts bearing bisphosphine ligands such as PdCl2(dppe), PdCl2(dppb) gave unsatisfactory results (entries 7,8). The use of a Pd2(dba)3 catalyst resulted in no reaction. Gratifyingly, PdCl2(PPh3)2 using cyclopentyl methyl ether (CPME) solvent furnished 5 in 51% isolated yield.

Table 1.
Screening of Pd catalysts for Miyaura–Ishiyama cross-coupling.
On the other hand, Miyaura–Ishiyama cross-coupling using chlobenthiazone instead for 5 with (pin)2B2 did not proceed due to lower reactivity of the chlorinated substrate.
2. Experimental Section
General
All reactions were carried out in oven-dried glassware under an argon atmosphere. Flash column chromatography was performed with silica gel Merck 60 (230–400 mesh ASTM). TLC analysis was performed on 0.25 mm Silicagel Merck 60 F254 plates. Melting points were determined on a hot stage microscope apparatus (ATM-01, AS ONE, Osaka, Japan) and were uncorrected. NMR spectra were recorded on a JEOL DELTA 300 or JEOLRESONANCE ECX-500 spectrometer (JEOL, Tokyo, Japan), operating at 300 MHz or 500 MHz for 1H-NMR, and at 75 MHz or 120 MHz for 13C-NMR. Chemical shifts (δ ppm) in CDCl3 were reported downfield from TMS (= 0) for 1H-NMR. For 13C-NMR, chemical shifts were reported relative to CDCl3 (77.00 ppm) as an internal reference. IR Spectra were recorded on a JASCO FT/IR-5300 spectrophotometer (JASCO Corporation, Tokyo, Japan). Mass spectra were measured on a JEOL JMS-T100LC spectrometer (JEOL, Tokyo, Japan).
2-Bromo-N-methylaniline (4) [29]
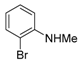 n-BuLi (1.60 M in hexane, 13.0 mL, 20.0 mmol) was added to a stirred solution of 2-bromoaniline (3.44 g, 20.0 mmol) in THF (20 mL) at −78 °C under an Ar atmosphere, followed by stirring at same temperature for 15 min. MeI (1.3 mL, 20.0 mmol) was slowly added at that temperature and the mixture was allowed to warm to 20–25 °C. Stirring continued at same temperature for an additional 20 h. Water was added to the stirred mixture, which was extracted twice with ethyl acetate (AcOEt). The organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The resulting crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 10:1) to give the desired product (3.54 g, 90%).
n-BuLi (1.60 M in hexane, 13.0 mL, 20.0 mmol) was added to a stirred solution of 2-bromoaniline (3.44 g, 20.0 mmol) in THF (20 mL) at −78 °C under an Ar atmosphere, followed by stirring at same temperature for 15 min. MeI (1.3 mL, 20.0 mmol) was slowly added at that temperature and the mixture was allowed to warm to 20–25 °C. Stirring continued at same temperature for an additional 20 h. Water was added to the stirred mixture, which was extracted twice with ethyl acetate (AcOEt). The organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The resulting crude oil was purified by SiO2 column chromatography (hexane/AcOEt = 10:1) to give the desired product (3.54 g, 90%).

Yellow pale oil; 1H-NMR (500 MHz, CDCl3): δ = 2.89 (s, 3H), 4.34 (s, 1H), 6.56–6.59 (m, 1H), 6.61–6.63 (m, 1H), 7.19–7.22 (m, 1H), 7.40–7.42 (m, 1H); 13C-NMR (125 MHz, CDCl3): δ = 30.53, 109.5, 110.6, 117.5, 128.5, 132.2, 145.9.
4-Bromo-3-methylbenzo[d]thiazol-2(3H)-one (5) [4,5]
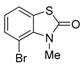 Chlorocarbonylsulfenyl chloride (CCSC; 2) (0.90 mL, 11.0 mmol) was added to a stirred solution of 2-bromo-N-methylaniline (1.86 g, 10.0 mmol) and N,N-dimethylaniline (1.33 g, 11.0 mmol) in toluene (10 mL) at 0–5 °C. Stirring continued at same temperature for 1 h under an Ar atmosphere. The reaction mixture was filtered through celite to remove HCl salt of N,N-dimethylaniline, and the filtrate was added to a stirred suspension of AlCl3 (2.00 g, 15.0 mmol) in toluene (10 mL) at room temperature. The mixture was refluxed for 3 h. After cooling to room temperature, water was added to the stirred mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude product was purified by SiO2 column chromatography (hexane/AcOEt = 10:1) to give the crude solid. Recrystallization from 2-propanol gave the desired product (1.31 g, 54%).
Chlorocarbonylsulfenyl chloride (CCSC; 2) (0.90 mL, 11.0 mmol) was added to a stirred solution of 2-bromo-N-methylaniline (1.86 g, 10.0 mmol) and N,N-dimethylaniline (1.33 g, 11.0 mmol) in toluene (10 mL) at 0–5 °C. Stirring continued at same temperature for 1 h under an Ar atmosphere. The reaction mixture was filtered through celite to remove HCl salt of N,N-dimethylaniline, and the filtrate was added to a stirred suspension of AlCl3 (2.00 g, 15.0 mmol) in toluene (10 mL) at room temperature. The mixture was refluxed for 3 h. After cooling to room temperature, water was added to the stirred mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water, brine, dried (Na2SO4) and concentrated. The obtained crude product was purified by SiO2 column chromatography (hexane/AcOEt = 10:1) to give the crude solid. Recrystallization from 2-propanol gave the desired product (1.31 g, 54%).

Colorless crystals; mp 130–132 °C; 1H-NMR (500 MHz, CDCl3): δ =3.87 (s, 3H), 6.97–7.00 (m, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3): δ = 33.40, 104.0, 121.6, 123.7, 124.9, 132.4, 134.8, 170.0; IR (neat): νmax = 1450, 1435, 1311, 1265, 1257, 1211, 1193, 1149, 1128, 1093, 1072 cm−1; HRMS (ESI): m/z calcd. for C8H6BrNOS [M + Na]+ 243.9432; found: 243.9405.
3-Methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2(3H)-benzothiazolone (3)
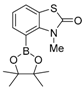 Bis(pinacolato)diboron ((pin)2B2) (190 mg, 0.75 mmol), KOAc (74 mg, 0.75 mmol), and bis(triphenylphosphine)palladium(II) dichloride (20 mg, 0.5 mmol) were successively added to a stirred suspension of 4-bromo-3-methylbenzothiazol-2-one 5 (122 mg, 0.50 mmol) in CPME at 20–25 °C under an N2 atmosphere, and the mixture was stirred at 110 °C for 20 h. Water was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water and brine, dried, and concentrated. The crude product was purified by silica gel column chromatography (hexane/AcOEt = 5:1), and washed with hexane to give the desired product (75 mg, 51%).
Bis(pinacolato)diboron ((pin)2B2) (190 mg, 0.75 mmol), KOAc (74 mg, 0.75 mmol), and bis(triphenylphosphine)palladium(II) dichloride (20 mg, 0.5 mmol) were successively added to a stirred suspension of 4-bromo-3-methylbenzothiazol-2-one 5 (122 mg, 0.50 mmol) in CPME at 20–25 °C under an N2 atmosphere, and the mixture was stirred at 110 °C for 20 h. Water was added to the mixture, which was extracted twice with AcOEt. The combined organic phase was washed with water and brine, dried, and concentrated. The crude product was purified by silica gel column chromatography (hexane/AcOEt = 5:1), and washed with hexane to give the desired product (75 mg, 51%).

Colorless crystals; mp 98–100 °C; 1H-NMR (500 MHz, CDCl3): δ = 1.40 (s, 12 H), 3.60 (s, 3H), 7.13–7.16 (m, 1H), 7.46 (d, J = 7.5 Hz, 1H), 7.57 (d, 1H, J = 7.5 Hz) ; 13C-NMR (125 MHz, CDCl3): δ = 24.8, 32.1, 84.8, 122.4, 122.8, 124.6, 133.4, 141.1, 170.5.; IR (neat): νmax = 1591, 1404, 1141, 1109, 1078, 1056, 1008, 960, 869, 846 cm−1; HRMS (ESI): m/z calcd. for C14H18BNO3S, [M + Na]+ 314.1001; found: 314.1000.
3. Conclusions
Straightforward synthesis of a novel 4-(pinacolato)borane [(pin)B] derivative of N-methyl-2(3H)-benzothiazol-2-one was performed through two key steps: (1) cyclo-condensation of N-methyl-2-bromoaniline with chlorocarbonylsulfenyl chloride (CCSC) to give N-methyl-2-bromo-2(3H)-benzothiazol-2-one; and (2) Miyaura–Ishiyama cross-coupling of this intermediate to produce 3-methyl-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2(3H)-benzothiazol-2-one.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2018/1/M976/s1. All materials (substrates and reagents) in this work are commercially available at an inexpensive price. Copies of the 1H, and 13C-NMR spectra for compounds 3, 4, and 5 are available in the supplementary information.
Acknowledgments
This research was partially supported by Grant-in-Aids for Scientific Research on Basic Areas (B) “18350056” and (C) 15K05508, Priority Areas (A) “17035087” and “18037068”, and Exploratory Research “17655045” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). One of the authors (Y.T.) offers his warmest congratulations to Ben L. Feringa (University of Groningen, The Netherlands) on being awarded the 2016 Nobel Prize in Chemistry. A dedication is made to Teruaki Mukaiyama on the celebration of his 90th birthday (Sotuju).
Author Contributions
S.I. contributed the overall syntheses. Y.T. prepared the whole manuscript. H.N. assisted in the references.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elderfield, R.C. Heterocyclic Compounds; John Wiley & Sons: New York, NY, USA, 1957; p. 484. [Google Scholar]
- Folco, G.C.; Vigano, T.; Sautebin, L.; Malandrinno, S.; Omini, C.; Berti, F. The mode of action of tiaramide hydrochloride: A new anti-asthmaticdrug. Pharmacol. Res. Commun. 1979, 11, 703–718. [Google Scholar] [CrossRef]
- Godson, D.H.; Leafe, E.L.; Lush, G.B.; Stevenson, H.A. Herbicidal 2-benzothiazolinone derivatives. GB862226, 8 March 1961. [Google Scholar]
- Uematsu, T.; Inoue, S.; Yamashita, N. Benzazole Derivatives. Jpn. Patent 54,041,870, 3 April 1979. [Google Scholar]
- Inoue, S.; Uematsu, T.; Kato, T.; Ueda, K. New melanin biosynthesis inhibitors and their structural similarities. Pest Manag. Sci. 1985, 16, 589–598. [Google Scholar] [CrossRef]
- Shin-ya, K.; Umeda, Y.; Chijiwa, S.; Furihata, K.; Furihata, K.; Hayakawa, Y.; Seto, H. Mevashuntin, a novel metabolite produced by inhibition of the mevalonate pathway in Streptomyces prunicolor. Tetrahedron Lett. 2005, 46, 1273. [Google Scholar] [CrossRef]
- Nawrat, C.C.; Moody, C.J. Total Synthesis of Mevashuntin. Org. Lett. 2012, 14, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.W.; Terefenko, E.A.; Wrobel, J.; Zhang, Z.M.; Zhu, Y.; Cohen, J.; Marschke, K.B.; Mais, D. Synthesis and progesterone receptor antagonist activities of 6-aryl benzimidazolones and benzothiazolones. Bioorg. Med. Chem. Lett. 2001, 11, 2747–2750. [Google Scholar] [CrossRef]
- Ucar, H.; Van derpoorten, K.; Cacciaguerra, S.; Spampinato, S.; Stables, J.P.; Depovere, P.; Isa, M.; Masereel, B.; Delarge, J.; Poupaert, J.H. Synthesis and anticonvulsant activity of 2(3H)-benzoxazolone and 2(3H)-benzothiazolone derivatives. J. Med. Chem. 1998, 41, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Yous, S.; Wallez, V.; Belloir, M.; Caignard, D.H.; McCurdy, C.R.; Poupaert, J.H. Novel 2(3H)-benzothiazolones as highly potent and selective sigma-1 receptor ligands. Med. Chem. Res. 2005, 14, 158–168. [Google Scholar] [CrossRef]
- Weng, J.Q.; Liu, X.H.; Huang, H.; Tan, C.X.; Chen, J. Synthesis, structure and antifungal activity of new 3-[(5-aryl-1,3,4-oxadiazol-2-yl)methyl]benzo[d]thiazol-2(3H)-ones. Molecules 2012, 17, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Muehlbauer, E.; Weiss, W. Process for the Preparation of Substituted 1,3-Oxathiol-2-ones. German Patent 1,233,882, 18 March 1965. [Google Scholar]
- Zumack, V.G.; Kühle, E. Chlorosulfenylated carbonic acid derivatives. Angew. Chem. Int. Ed. 1970, 82, 54–63. [Google Scholar] [CrossRef]
- Tanabe, Y.; Okabe, T.; Kakimizu, A.; Ohno, N.; Yoshioka, H. An improved method for preparation of N-alkyl-2(3H)-benzothiazolone. Bull. Chem. Soc. Jpn. 1983, 56, 1255–1256. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sanemitsu, Y. A covenient synthesis of 3-chloromethyl-2(3H)-benzothiazolone. Synthesis 1988, 482–484. [Google Scholar] [CrossRef]
- Sanemitsu, Y.; Kawamura, S.; Tanabe, Y. Regioselective α-methoxycarbonyl-sulfenylation of ketones and aldehydes: A versatile method for preparation of thiazolones, thiadiazinones, and 3-indolethiols. J. Org. Chem. 1992, 57, 1053–1056. [Google Scholar] [CrossRef]
- Tanabe, Y.; Mori, K.; Nishii, Y. Regioselective cyclocondensations of chlorocarbonylsulfenyl chloride with hydrazones: Effective synthesis of a class of sulfur and nitrogen containing heterocycles with -COS- linkage. Heterocycles 1996, 43, 141–149. [Google Scholar] [CrossRef]
- Ashida, Y.; Sato, Y.; Suzuki, T.; Ueno, K.; Kai, K.; Nakatsuji, H.; Tanabe, Y. (E)-, (Z)-parallel preparative methods for stereodefined β,β-Diaryl- and α,β-diaryl-α,β-unsaturated esters: Application to stereocomplementary concise synthesis of zimelidine. Chem. Eur. J. 2015, 21, 5934–5945. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Ashida, Y.; Hori, H.; Sato, Y.; Honda, A.; Taira, M.; Tanabe, Y. (E)- and (Z)-stereodefined enol phosphonates derived from β-ketoesters: Stereocomplementary synthesis of fully-substituted α,β-unsaturated esters. Org. Biomol. Chem. 2015, 13, 8205–8210. [Google Scholar] [CrossRef] [PubMed]
- Ashida, Y.; Nakatsuji, H.; Tanabe, Y. (Z)-Enol p-Tosylate Derived from Methyl Acetoacetate: A Useful Cross-coupling Partner for the Synthesis of Methyl (Z)-3-Phenyl (or Aryl)-2-butenoate. Org. Synth. 2017, 94, 93–108. [Google Scholar] [CrossRef]
- Ashida, Y.; Honda, A.; Sato, Y.; Nakatsuji, H.; Tanabe, Y. Divergent synthetic access to (E)- and (Z)-stereodefined all-carbon (fully)-substituted olefin scaffolds: Application to parallel synthesis of both (E)- and (Z)-tamoxifens. ChemistryOpen 2017, 6, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, H.; Kamada, R.; Kitaguchi, H.; Tanabe, Y. Dehydration-type Ti-claisen condensation (carbonhomologation) of α-heteroatom-substituted acetates with alkyl formates: Utilization as (Z)-stereodefined cross-coupling partners and application to concise synthesis of strobilurin A. Adv. Synth. Catal. 2017, 359, 3865–3879. [Google Scholar] [CrossRef]
- Neya, M.; Yamazaki, H.; Ohne, K.; Sawada, Y.; Mizutani, T.; Imamura, Y.; Mukai, N. Thiazepinyl Hydroxamic Acid Derivatives as Matrix Metalloproteinase Inhibitors. PCT Int. Appl. WO2001060808, 20 February 2001. [Google Scholar]
- Oku, T.; Hirayama, Y.; Yamagami, K.; Ohkubo, Y.; Matsuoka, H. Preparation of Substituted 2-(1,1-Dioxoperhydro-1,4-thiazepin-7-yl)acetamides for Treating Inflammatory Respiratory Diseases. PCT Int. Appl. WO2003018019, 6 March 2003. [Google Scholar]
- Adams, C.M.; Chamoin, S.; Hu, Q.Y.; Zhang, C. Preparation of 5-(Pyridin-3-yl)-1,3-dihydroindol-2-one Derivatives as Modulators of Aldosterone Synthase and/or CYP11B1. PCT Int. Appl. WO2010130794, 18 November 2010. [Google Scholar]
- Kaneko, S.; Sato, K.; Shikanai, D.; Yamada, R.; Sakurada, K. Preparation of 8-Substituted Isoquinoline Derivatives as IKKβ Kinase Inhibitors. PCT Int. Appl. WO2010038465, 8 April 2010. [Google Scholar]
- Fananas, F.J.; Granados, A.; Sanz, R.; Ignacio, J.M.; Barluenga, J. Synthesis of functionalized pyrrole and indole derivatives through carbometallation of lithiated double bonds. Chem. Eur. J. 2001, 7, 2896–2907. [Google Scholar] [CrossRef]
- Ishiyama, T.; Miyaura, N. Metal-catalyzed reactions of diborons for synthesis of organoboron compounds. Chem. Rec. 2004, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sheinin, E.B.; Bell, C.L.; Bauer, L. N-(9-o-carbazolyl)-haloanilines from the decompositions of o-carboxybenzenediazonium halides involving benzyne intermediates. J. Heterocycl. Chem. 1975, 12, 203–206. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).