5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene
Abstract
:1. Introduction
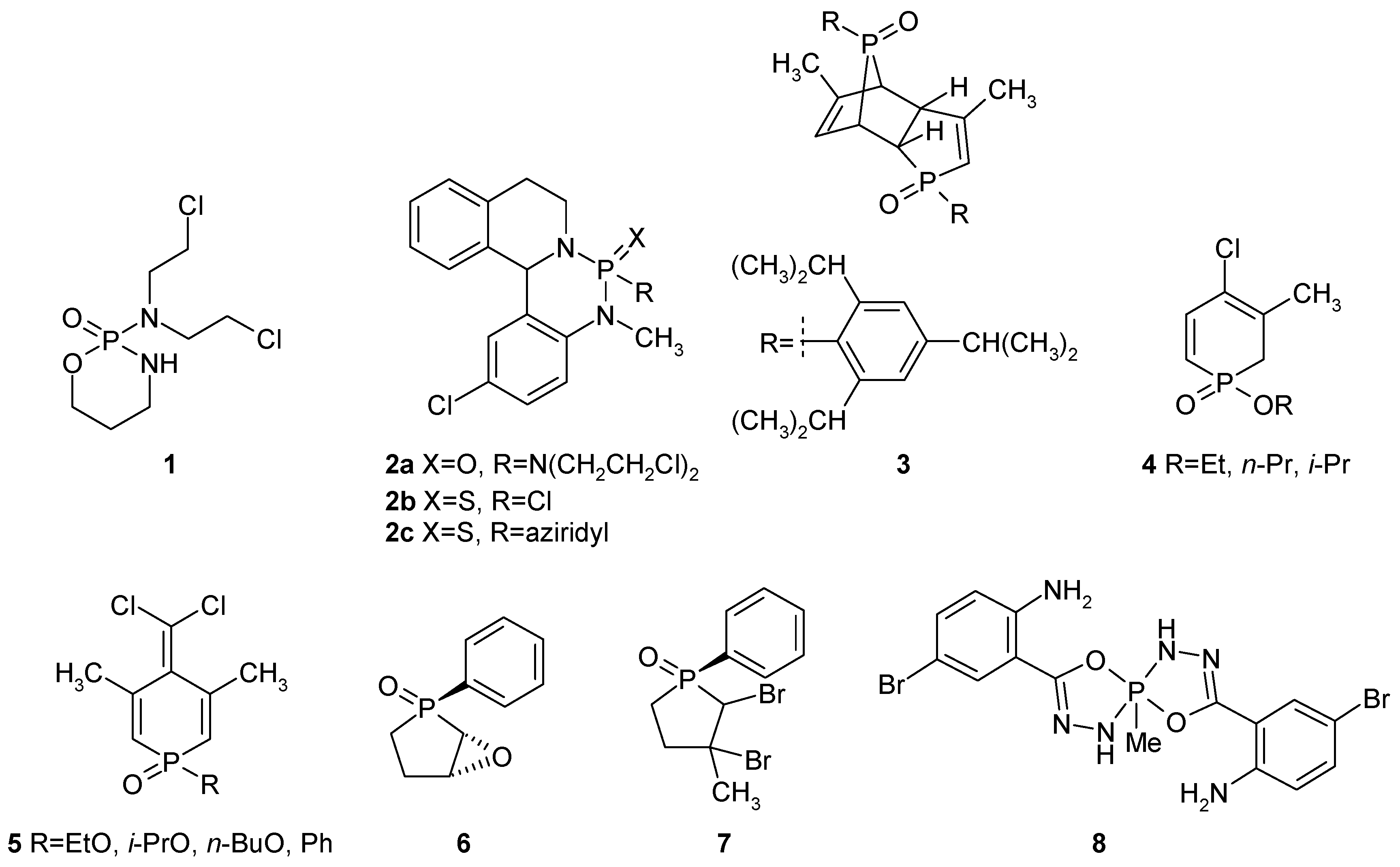
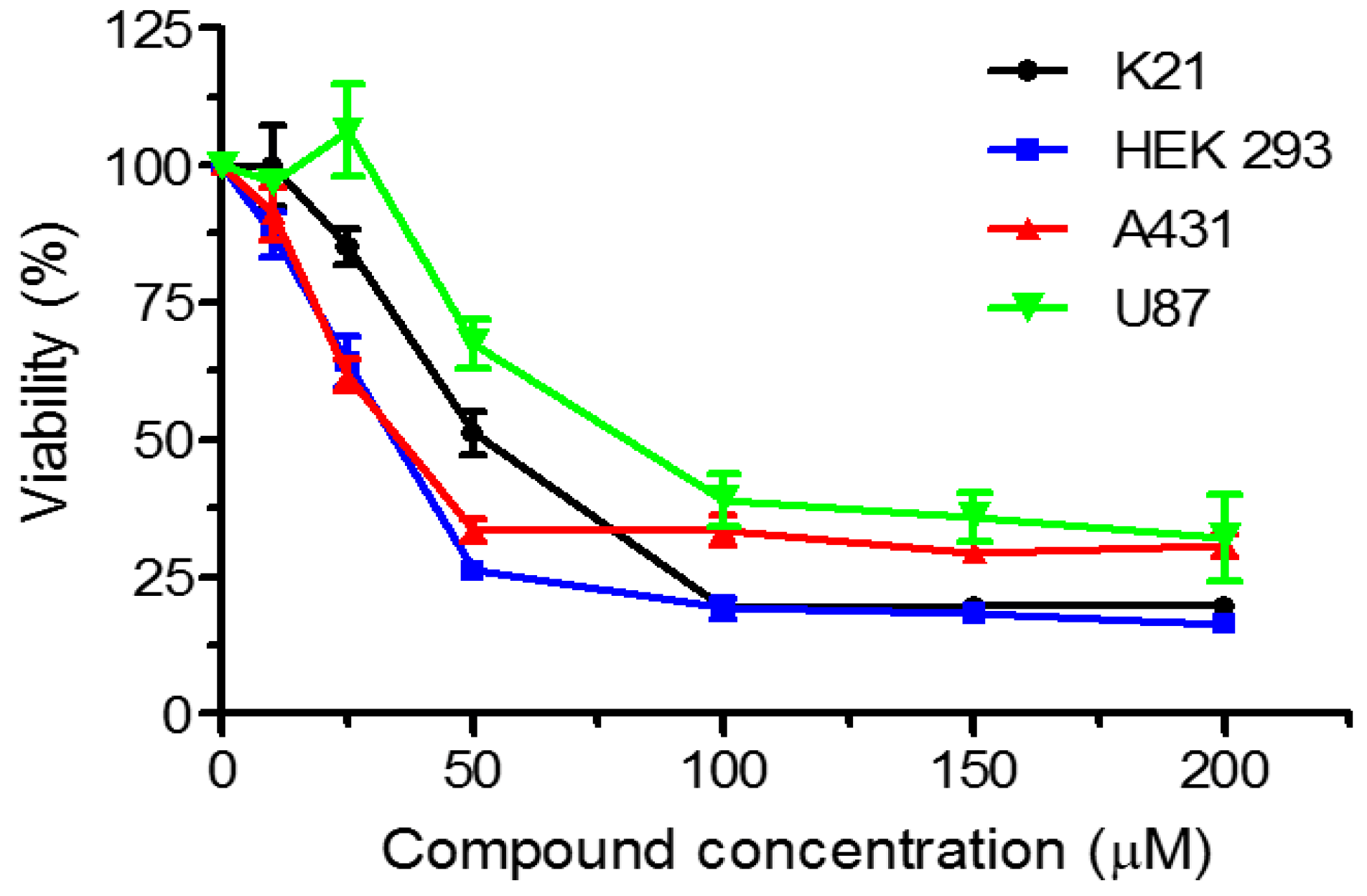
2. Results and Discussion
3. Materials and Methods
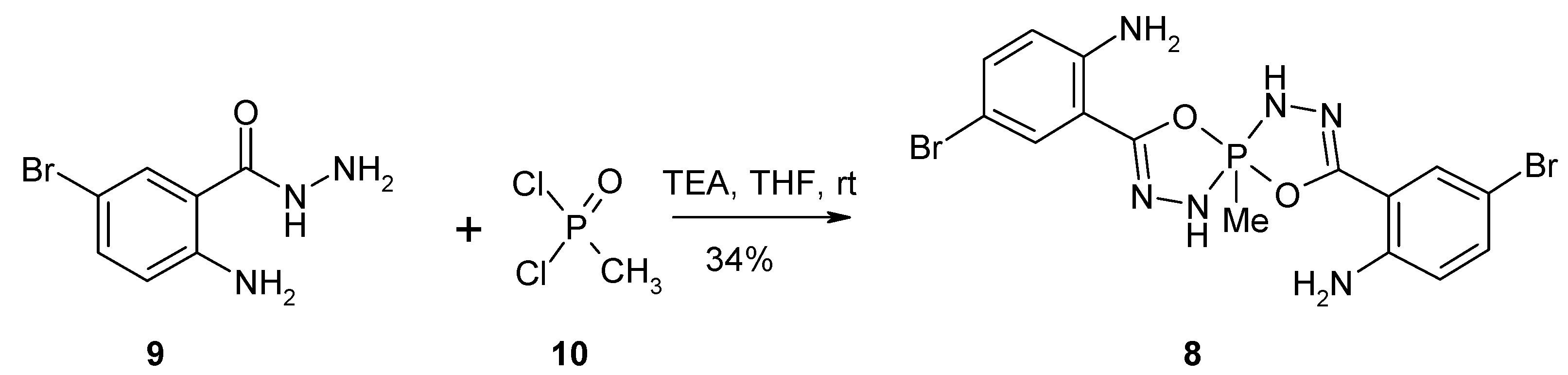
Synthesis of 5-methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene 8.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, A.R.; Hombal, S.M. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J. Am. Acad. Dermatol. 1984, 11, 1115–1126. [Google Scholar] [CrossRef]
- Bull, E.O.J.; Naidu, M.S.R. Isoquino[2,1-c][1,3,2]benzodiazaphosphorine derivatives: New potential agents for cancer chemotherapy. Phosphorus Sulfur Silicon Relat. Elem. 2000, 162, 231–243. [Google Scholar] [CrossRef]
- Hudson, H.R.; Keglevich, G. The preparation and anticancer activity of some phosphorus heterocycles. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2256–2261. [Google Scholar] [CrossRef]
- Ito, S.; Yamashita, M.; Niimi, T.; Fujie, M.; Reddy, V.K.; Totsuka, H.; Haritha, B.; Maddali, K.; Nakamura, S.; Asai, K.; et al. Preparation and characterization of phospholanes and phosphasugars as novel anti-cancer agents. Heterocycl. Commun. 2009, 15, 23–30. [Google Scholar] [CrossRef]
- Hasegawa, H.; Yamashita, M.; Makita, R.; Yamaoka, M.; Fujie, M.; Nakamura, S.; Oshikawa, T.; Yamashita, J.; Yamada, M.; Kondo, M.; et al. Novel molecular targeted and wide spectrum antitumor agents: Preparation and preclinical evaluation of IER5/Cdc25B targeted low-molecular-weight phospha sugar derivatives. JJAP Conf. Proc. 2016, 4, 011301. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Bazlekova, M.; Bagiński, M.; Wójcik, J.; Winczura, A.; Miazga, A.; Gajda, R.; Woźniak, K.; Tudek, B. A mild and efficient approach to 6H-oxazolo[3,2-f]pyrimidine-5,7-dione scaffold via unexpected rearrangement of 2,3-dihydropyrimido[6,1-b][1,5,3]dioxazepine-7,9(5H,8H)-diones: A synthesis, crystallographic studies and cytotoxic activity screening. Tetrahedron Lett. 2016, 57, 743–746. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Trzebiński, D.; Wilczek, M.; Psurski, M.; Bagiński, M.; Bieszczad, B.; Mroczkowska, M.; Woźniak, K. (S)-2-(4-Chlorobenzoyl)-1,2,3,4-tetrahydrobenzo[e]pyrazino[1,2-a][1,4]diazepine-6,12(11H,12aH)-dione—Synthesis and Crystallographic Studies. Molbank 2017, 2017, M964. [Google Scholar] [CrossRef]
- Mieczkowski, A.; Wińska, P.; Kaczmarek, M.; Mroczkowska, M.; Garbicz, D.; Pilżys, T.; Marcinkowski, M.; Piwowarski, J.; Grzesiuk, E. 2′-Deoxy-2′-azidonucleoside analogues: Synthesis and evaluation of antitumor and antimicrobial activity. Chem. Pap. 2017. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Galli, A.; Costagli, C. Synthesis and Biological Evaluation of a Series of Quinazoline-2-carboxylic Acids and Quinazoline-2,4-diones as Glycine-NMDA Antagonists: A Pharmacophore Model Based Approach. Arch. Pharm. 1997, 330, 129–134. [Google Scholar] [CrossRef]
- Schmidpeter, A.; Luber, J. Die produkte der Reaktion Carbonsäurehydrazid/Phosphonylchlorid: 2,2′-spirobi(1,3,4,2λ5-oxadiazaphospholine). Chem. Ber. 1977, 110, 1124–1129. [Google Scholar] [CrossRef]
- Gholivand, K.; Mahzouni, H.R.; Molami, F.; Kalateh, A.A. A phosphoryl to spiro-bicyclophosphorane transformation via β-amidic proton elimination in phosphorylated hydrazides. Tetrahedron Lett. 2012, 53, 5944–5947. [Google Scholar] [CrossRef]
- Hua, G.; Cordes, D.B.; Li, Y.; Slawin, A.M.Z.; Woollins, J.D. Symmetrical spiro-phosphoroheterocycles from selenation of carbohydrazides. Tetrahedron Lett. 2011, 52, 3311–3314. [Google Scholar] [CrossRef]
- Ali, T.E.; Halacheva, S.S. Synthetic Approach for Novel Bis(α-aminophosphonic acid) Derivatives of Chromone Containing 1,2,4,3-Triazaphosphole Moieties. Heteroat. Chem. 2009, 20, 117–122. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasperowicz, S.; Czerwińska, J.; Majchrzak, B.; Tudek, B.; Mieczkowski, A. 5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene. Molbank 2018, 2018, M978. https://doi.org/10.3390/M978
Kasperowicz S, Czerwińska J, Majchrzak B, Tudek B, Mieczkowski A. 5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene. Molbank. 2018; 2018(1):M978. https://doi.org/10.3390/M978
Chicago/Turabian StyleKasperowicz, Sławomir, Jolanta Czerwińska, Bartosz Majchrzak, Barbara Tudek, and Adam Mieczkowski. 2018. "5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene" Molbank 2018, no. 1: M978. https://doi.org/10.3390/M978
APA StyleKasperowicz, S., Czerwińska, J., Majchrzak, B., Tudek, B., & Mieczkowski, A. (2018). 5-Methyl-3,8-di-(2-amino-4-bromophenyl)-4,9-dioxa-1,2,6,7-tetraaza-5λ5-phosphaspiro[4.4]nona-2,7-diene. Molbank, 2018(1), M978. https://doi.org/10.3390/M978




