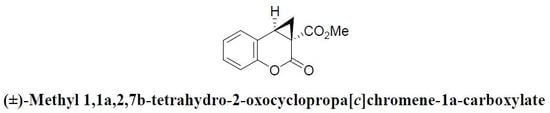
(±)-Methyl 1,1a,2,7b-Tetrahydro-2-oxocyclopropa[c] Chromene-1a-carboxylate
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Syntheis of (±)-Methyl 1,1a,2,7b-Tetrahydro-2-oxocyclopropa[c]chromene-1a-carboxylate (3)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donaldson, W.-A. Synthesis of cyclopropane containing natural products. Tetrahedron 2003, 57, 8587–8627. [Google Scholar] [CrossRef]
- Brackmann, F.; Meijere, A. Natural occurrence, syntheses, and applications of cyclopropyl-group-containing α-amino acids. 1. 1-Aminocyclopropanecarboxylic acid and other 2,3-methanoamino acids. Chem. Rev. 2007, 107, 4493–4537. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.-K.; Pouwer, R.H.; Richard, J.-A. Recent advances in the total synthesis of cyclopropane-containing natural products. Chem. Soc. Rev. 2012, 41, 4631–4642. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, G.; Bencivenni, G.; Dalpozzo, R. Asymmetric cyclopropanation reactions. Synthesis 2014, 46, 979–1029. [Google Scholar]
- Reissig, H.-U.; Zimmer, R. Donor–acceptor-substituted cyclopropane derivatives and their application in organic synthesis. Chem. Rev. 2003, 103, 1151–1196. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A new golden age for donor-acceptor cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Kim, S.-G. Lewis acid-catalyzed Friedel–Crafts alkylation of donor–acceptor cyclopropanes with electron-rich benzenes to generate 1,1-diarylalkanes. Eur. J. Org. Chem. 2015, 6419–6422. [Google Scholar] [CrossRef]
- Sin, S.; Kim, S.-G. Stereoselective cascade reactions of donor–acceptor cyclopropanes with m-N,N-dialkylaminophenyl α,β-unsaturated carbonyls: Facile diastereoselective synthesis of cis- and trans-tetralins. Adv. Synth. Catal. 2016, 358, 2701–2706. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, H.A.; Farhat, A.A. Ethyl coumarin-3-carboxylate: Synthesis and chemical properties. Org. Commun. 2014, 7, 1–27. [Google Scholar]
- Yamashita, M.; Okuyama, K.; Kawajiri, T.; Takada, A.; Inagaki, Y.; Nakano, H.; Tomiyama, M.; Ohnaka, A.; Terayama, I.; Kawasaki, I.; et al. A novel tandem reaction of 3-substituted coumarins with two equivalents of dimethylsulfoxonium ylide to 2-substituted cyclopenta[b]benzofuran-3-ol derivatives. Tetrahedron 2002, 58, 1497–1505. [Google Scholar] [CrossRef]
- Yamashita, M.; Okuyama, K.; Kawasaki, I.; Ohta, S. One-step synthesis of 2-substituted cyclopenta[b]benzofuran-3-ol derivatives from 3-substituted coumarins. Tetrahedron Lett. 1995, 36, 5603–5606. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Kim, S.-G. (±)-Methyl 1,1a,2,7b-Tetrahydro-2-oxocyclopropa[c] Chromene-1a-carboxylate. Molbank 2017, 2017, M966. https://doi.org/10.3390/M966
Choi S, Kim S-G. (±)-Methyl 1,1a,2,7b-Tetrahydro-2-oxocyclopropa[c] Chromene-1a-carboxylate. Molbank. 2017; 2017(4):M966. https://doi.org/10.3390/M966
Chicago/Turabian StyleChoi, Sunyoung, and Sung-Gon Kim. 2017. "(±)-Methyl 1,1a,2,7b-Tetrahydro-2-oxocyclopropa[c] Chromene-1a-carboxylate" Molbank 2017, no. 4: M966. https://doi.org/10.3390/M966
APA StyleChoi, S., & Kim, S.-G. (2017). (±)-Methyl 1,1a,2,7b-Tetrahydro-2-oxocyclopropa[c] Chromene-1a-carboxylate. Molbank, 2017(4), M966. https://doi.org/10.3390/M966