Heterobimetallic 3d-4f (Zn/La) {6,6′-Dimethoxy-2,2′-[naphthalene-2,3-diylbis(nitrilomethylidyne)]diphenolato}-pyridylzinc(II)-tris(nitrato-O,O′)lanthanum(III) Monohydrate
Abstract
:1. Introduction
2. Results and Discussion
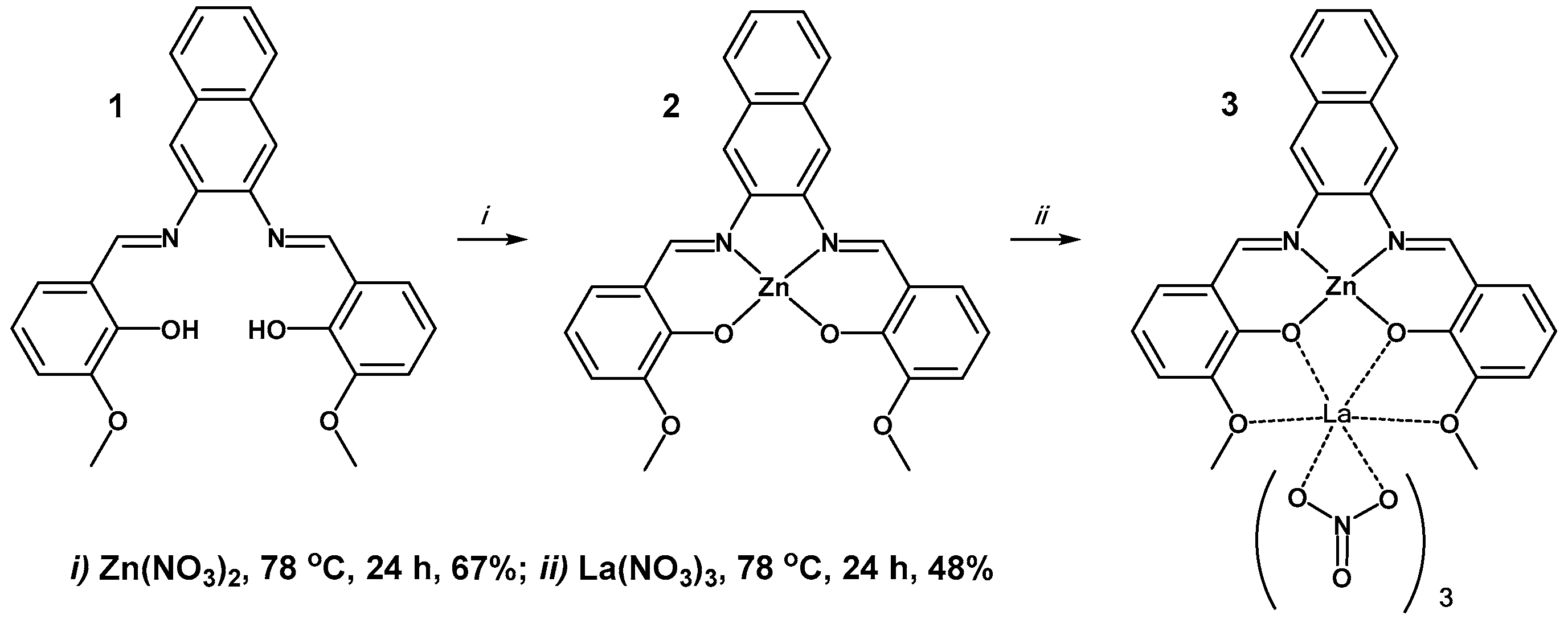
2.1. Synthetic Details
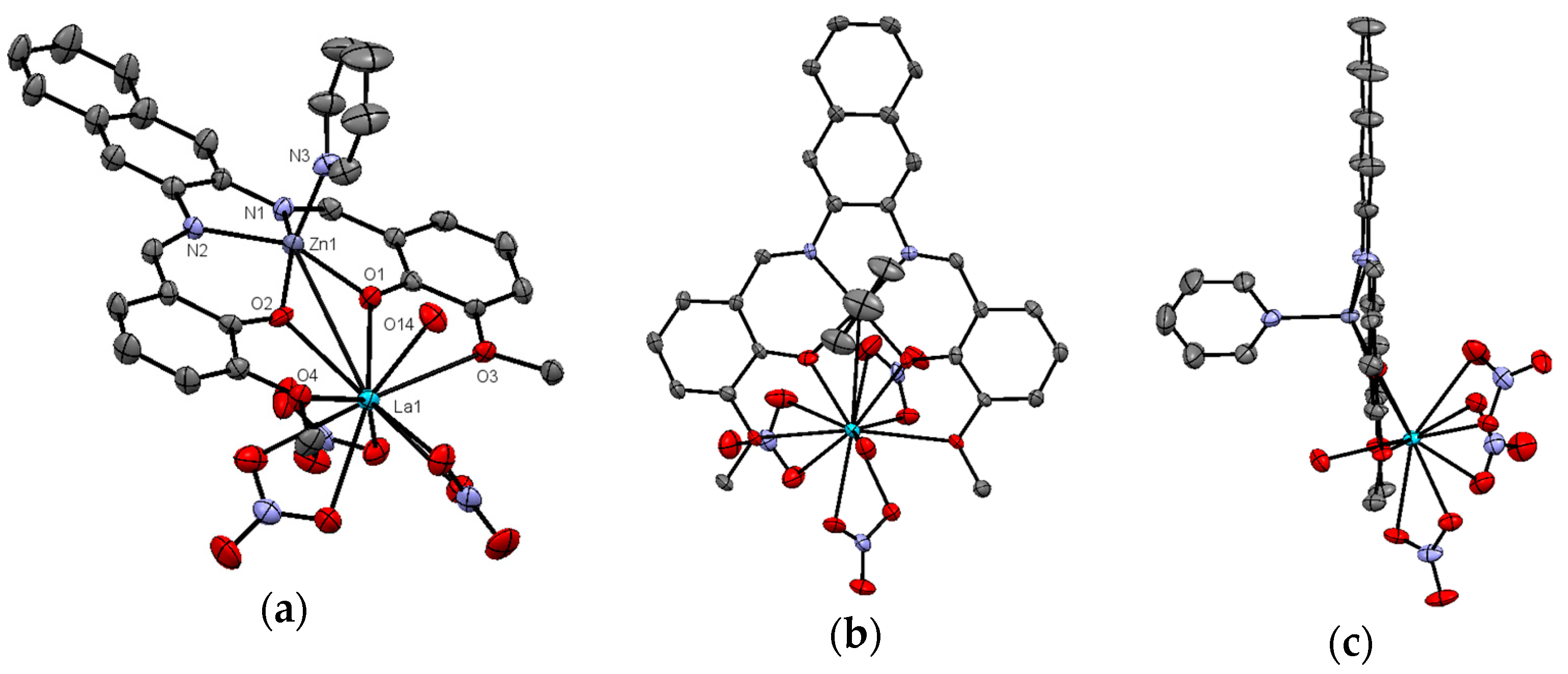
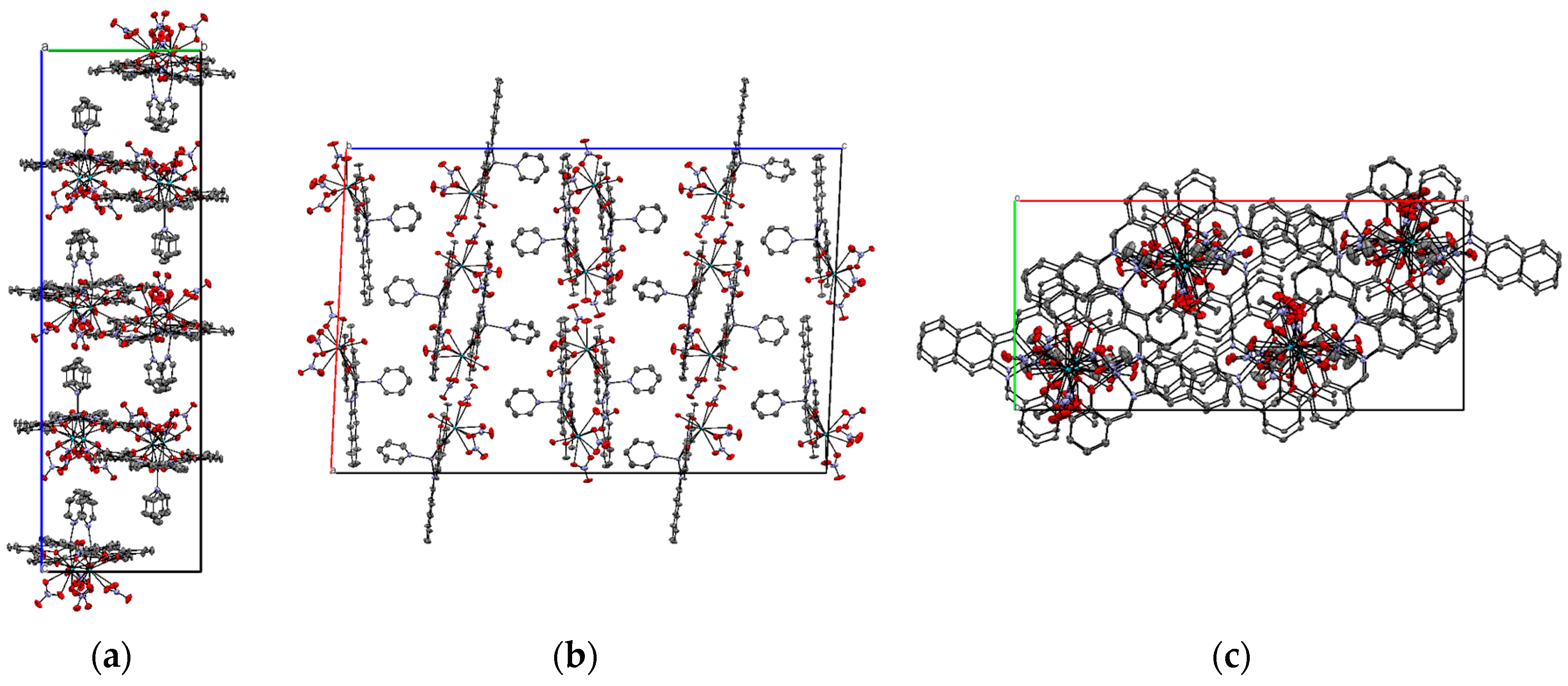
2.2. X-ray Structure of Zn2+/La2+ Complex (3)
3. Materials and Methods
3.1. General Considerations
3.2. Synthetic Details
3.3. X-ray Diffraction Studies
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Cozzi, P.G. Metal-salen schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.S.; Jacobsen, E.N. Enantioselective Addition of Hydrogen Cyanide to imines Catalyzed by a Chiral (Salen)Al(III) Complex. J. Am. Chem. Soc. 1998, 120, 5315–5316. [Google Scholar] [CrossRef]
- White, D.E.; Tadross, P.M.; Lu, Z.; Jacobsen, E.N. A broadly applicable and practical oligomeric (salen)Co catalyst for enantioselective epoxide ring-opening reactions. Tetrahedron 2014, 70, 4165–4180. [Google Scholar] [CrossRef] [PubMed]
- Larrow, J.F.; Jacobsen, E.N. Asymmetric processes catalyzed by chiral (salen)metal complexes. Top. Organomet. Chem. 2004, 6, 123–152. [Google Scholar] [CrossRef]
- Hari, N.; Jana, A.; Mohanta, S. Syntheses, crystal structures and esi-ms of mononuclear-Dinuclear, trinuclear and dinuclear based one-dimensional copper(II)-s block metal ion complexes derived from a 3-ethoxysalicylaldehyde-diamine ligand. Inorg. Chim. Acta 2017, 467, 11–20. [Google Scholar] [CrossRef]
- Deng, X.-W.; Cai, L.-Z.; Zhu, Z.-X.; Gao, F.; Zhou, Y.-L.; Yao, M.-X. Synthesis, structures and magnetic properties of chiral 3d-3d′-4f heterotrimetallic complexes based on [(Tp*)Fe(CN)3]−. New J. Chem. 2017, 41, 5988–5994. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Su, P.; Zhang, Z.; Fu, G.; Li, B.; Lu, X. Red to white polymer light-emitting diode (PLED) based on Eu3+-Zn2+-Gd3+-containing metallopolymer. J. Mater. Chem. C 2017, 5, 4780–4787. [Google Scholar] [CrossRef]
- Pushkarev, A.P.; Balashova, T.V.; Kukinov, A.A.; Arsenyev, M.V.; Yablonskiy, A.N.; Kryzhkov, D.I.; Andreev, B.A.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Sensitization of NIR luminescence of Yb3+ by Zn2+ chromophores in heterometallic complexes with a bridging schiff-base ligand. Dalton Trans. 2017, 46, 10408–10417. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Fu, G.; Liu, L.; Feng, W.; Lü, X. Single-nodal linking for Zn2+-Nd3+-containing metallopolymer with efficient near-infrared (NIR) luminescence. Inorg. Chem. Commun. 2017, 83, 36–39. [Google Scholar] [CrossRef]
- Lu, X.Q.; Feng, W.X.; Hui, Y.N.; Wei, T.; Song, J.R.; Zhao, S.S.; Wong, W.Y.; Wong, W.K.; Jones, R.A. Near-infrared luminescent, neutral, cyclic Zn2Ln2 (Ln = Nd, Yb, and Er) complexes from asymmetric salen-type schiff base ligands. Eur. J. Inorg. Chem. 2010, 2714–2722. [Google Scholar] [CrossRef]
- Yang, X.P.; Jones, R.A.; Wu, Q.Y.; Oye, M.M.; Lo, W.K.; Wong, W.K.; Holmes, A.L. Synthesis, crystal structures and antenna-like sensitization of visible and near infrared emission in heterobimetallic Zn-Eu and Zn-Nd schiff base compounds. Polyhedron 2006, 25, 271–278. [Google Scholar] [CrossRef]
- Wong, W.-K.; Yang, X.; Jones, R.A.; Rivers, J.H.; Lynch, V.; Lo, W.-K.; Xiao, D.; Oye, M.M.; Holmes, A.L. Multinuclear luminescent schiff-base zn-nd sandwich complexes. Inorg. Chem. 2006, 45, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.K.; Wong, W.K.; Wong, W.Y.; Guo, J.P.; Yeung, K.T.; Cheng, Y.K.; Yang, X.P.; Jones, R.A. Heterobimetallic Zn(II)-Ln(III) phenylene-bridged schiff base complexes, computational studies, and evidence for singlet energy transfer as the main pathway in the sensitization of near-infrared Nd3+ luminescence. Inorg. Chem. 2006, 45, 9315–9325. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Buenzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Ansari, K.I.; Grant, J.D.; Woldemariam, G.A.; Kasiri, S.; Mandal, S.S. Iron(III)-salen complexes with less DNA cleavage activity exhibit more efficient apoptosis in MCF7 cells. Org. Biomol. Chem. 2009, 7, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.I.; Grant, J.D.; Kasiri, S.; Woldemariam, G.; Shrestha, B.; Mandal, S.S. Manganese(iii)-salens induce tumor selective apoptosis in human cells. J. Inorg. Biochem. 2009, 103, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kuroda-Sowa, T.; Nabei, A.; Maekawa, M.; Okubo, T. {6,6′-dimethoxy-2,2′-[naphthalene-2,3-diylbis(nitrilomethylidyne)]diphenolato}thiocyanatocobalt(iii) diethyl ether dichloromethane solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m257–m258. [Google Scholar] [CrossRef] [PubMed]
- Average interatomic distance across four independent molecules in unit cell.
- Bruker. Apex3 v2016.9-0, SAINT V8.37A, Bruker AXS Inc.: Madison, WI, USA, 2013/2014.
- SHELXTL Suite of Programs, version 6.14; Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2000–2003.
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. University of Göttingen: Göttingen, Saxony, Germany. Personal communication, 2016. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hubschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. Wingx and ortep for windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
| Bond | A | B | C | D | Average 1 |
|---|---|---|---|---|---|
| Zn–N1 | 2.067 (6) | 2.061 (6) | 2.052 (6) | 2.058 (6) | 2.060 |
| Zn–N2 | 2.039 (7) | 2.049 (6) | 2.050 (6) | 2.048 (6) | 2.050 |
| Zn–N3 (pyridyl) | 2.052 (8) | 2.050 (19) | 2.056 (8) | 2.047 (7) | 2.044 |
| Zn–O1 | 1.998(5) | 1.998 (5) | 1.992 (5) | 2.005 (5) | 1.998 |
| Zn–O2 | 2.008 (5) | 2.004 (5) | 1.993 (5) | 1.984 (5) | 1.997 |
| La–O1 | 2.484 (5) | 2.466 (5) | 2.508 (5) | 2.513 (5) | 2.493 |
| La–O2 | 2.507 (5) | 2.518 (5) | 2.487 (5) | 2.488 (5) | 2.500 |
| La–O3 | 2.885 (6) | 2.996 (6) | 2.895 (6) | 3.04 (1) | 2.953 |
| La–O4 | 2.866 (6) | 2.875 (5) | 2.794 (5) | 2.856 (6) | 2.848 |
| La–O14 (aqua) | 2.504 (7) | 2.525 (6) | 2.504 (7) | 2.499 (6) | 2.508 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linden, C.B.S.; Chaudhary, N.; Zeller, M.; Trivedi, E.R. Heterobimetallic 3d-4f (Zn/La) {6,6′-Dimethoxy-2,2′-[naphthalene-2,3-diylbis(nitrilomethylidyne)]diphenolato}-pyridylzinc(II)-tris(nitrato-O,O′)lanthanum(III) Monohydrate. Molbank 2017, 2017, M965. https://doi.org/10.3390/M965
Linden CBS, Chaudhary N, Zeller M, Trivedi ER. Heterobimetallic 3d-4f (Zn/La) {6,6′-Dimethoxy-2,2′-[naphthalene-2,3-diylbis(nitrilomethylidyne)]diphenolato}-pyridylzinc(II)-tris(nitrato-O,O′)lanthanum(III) Monohydrate. Molbank. 2017; 2017(4):M965. https://doi.org/10.3390/M965
Chicago/Turabian StyleLinden, Calliope Bee Sue, Nikita Chaudhary, Matthias Zeller, and Evan R. Trivedi. 2017. "Heterobimetallic 3d-4f (Zn/La) {6,6′-Dimethoxy-2,2′-[naphthalene-2,3-diylbis(nitrilomethylidyne)]diphenolato}-pyridylzinc(II)-tris(nitrato-O,O′)lanthanum(III) Monohydrate" Molbank 2017, no. 4: M965. https://doi.org/10.3390/M965
APA StyleLinden, C. B. S., Chaudhary, N., Zeller, M., & Trivedi, E. R. (2017). Heterobimetallic 3d-4f (Zn/La) {6,6′-Dimethoxy-2,2′-[naphthalene-2,3-diylbis(nitrilomethylidyne)]diphenolato}-pyridylzinc(II)-tris(nitrato-O,O′)lanthanum(III) Monohydrate. Molbank, 2017(4), M965. https://doi.org/10.3390/M965





