Ethyl 4-[5-(methoxymethyl)furan-2-yl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Abstract
:1. Introduction
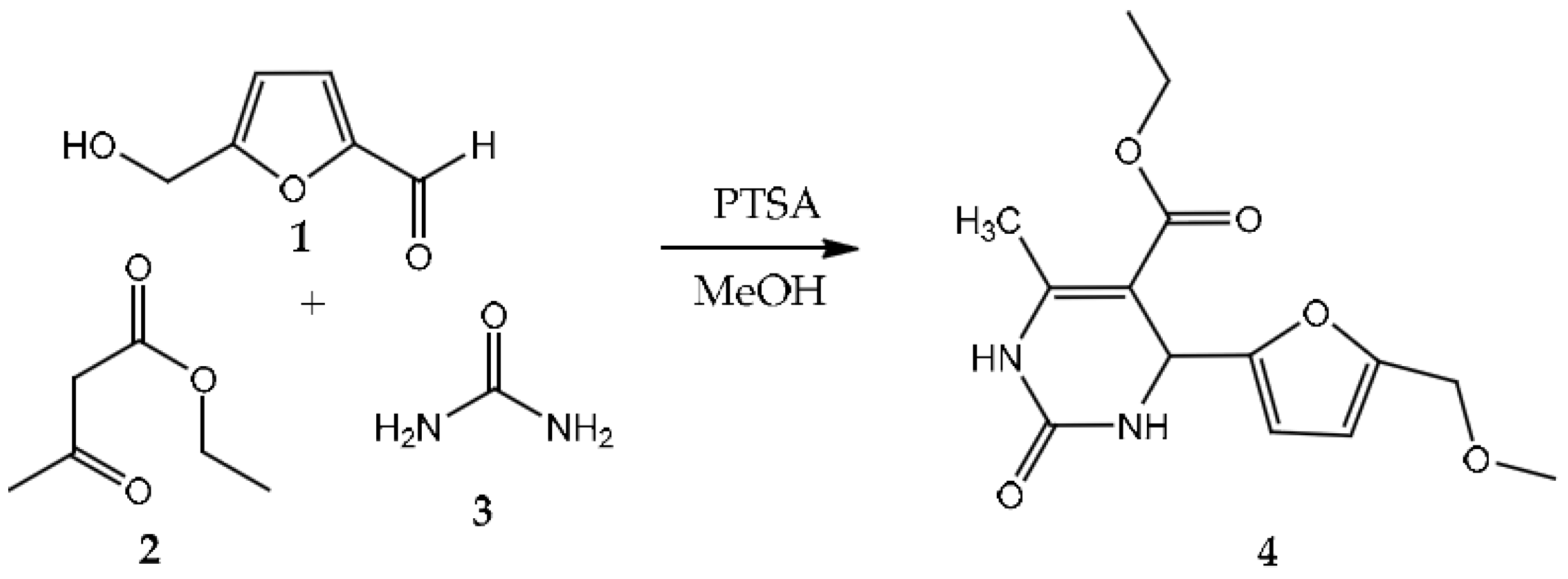
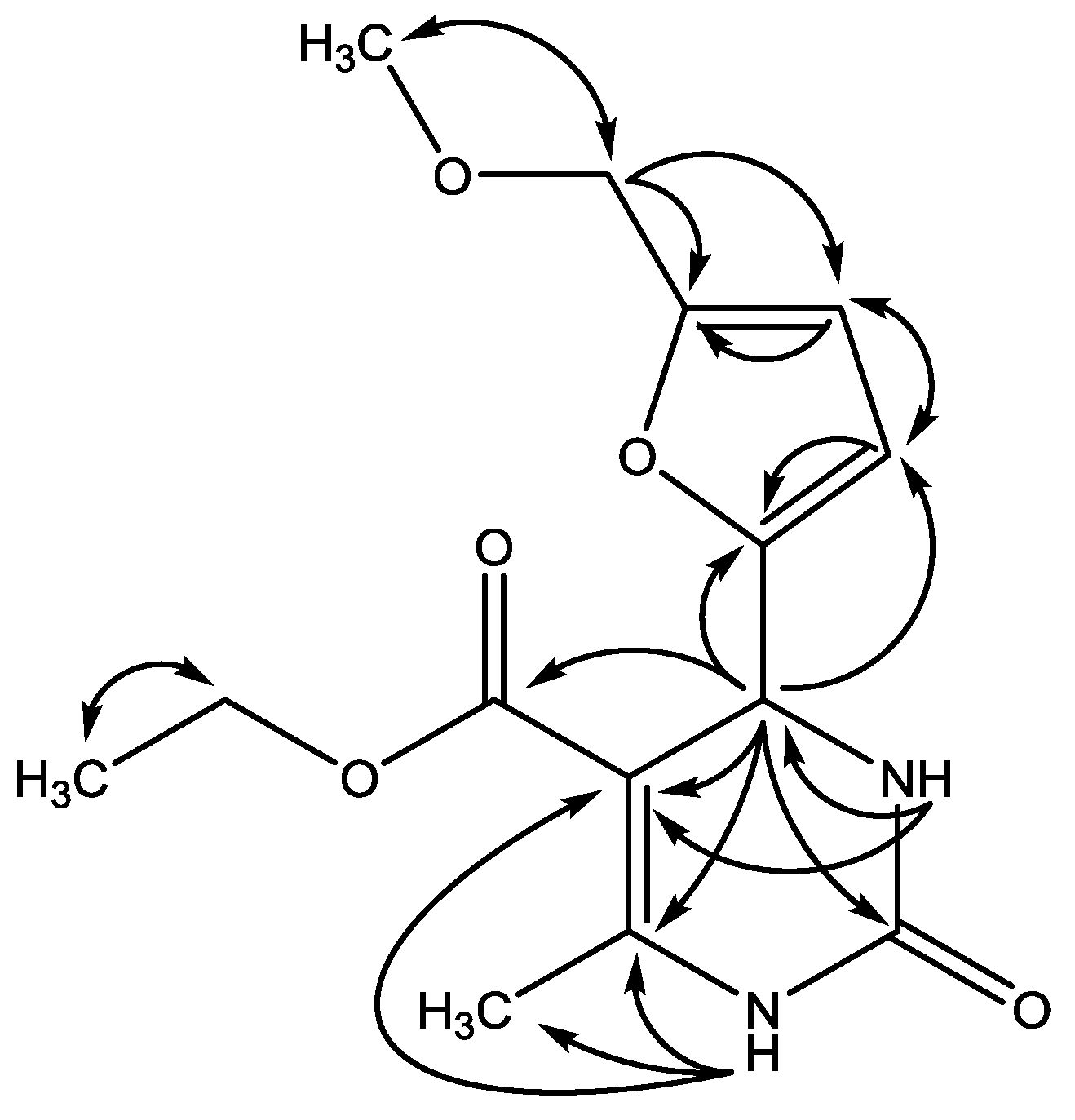
2. Results and Discussion
3. Materials and Methods
Synthesis Procedure of the Title Compound
4. Conclusion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biginelli, P. Derivati Aldeiduredici Degli Eteri Acetile Dossal-Acetico. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- De Vasconcelos, A.; Oliveira, P.S.; Ritter, M.; Freitag, A.; Romano, L.; Quina, F.H.; Pizzuti, L.; Pereira, C.M.P.; Stefanello, F.M.; Barschak, A.G. Antioxidant capacity and environmentally friendly synthesis of dihydropyrimidin-(2H)-ones promoted by naturally occurring organic acids. J. Biochem. Mol. Toxicol. 2012, 26, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bhatewara, A.; Jetti, S.R.; Kadre, T.; Paliwal, P.; dan Jain, S. Microwave-assisted synthesis and biological evaluation of dihydropyrimidinone derivatives as anti-inflammatory, antibacterial, and antifungal agents. Int. J. Med. Chem. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Ok, T.; So, W.; Jo, M.; Seo, M.; Kim, Y.; Sohn, J.H.; Park, Y.; Ju, M.K.; et al. Discovery of 3,4-dihydropyrimidin-2(1H)-ones with inhibitory activity against HIV-1 replication. Bioorg. Med. Chem. Lett. 2012, 22, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Sośnicki, J.G.; Struk, Ł.; Kurzawski, M.; Perużyńska, M.; Maciejewska, G.; dan Droździk, M. Regioselective synthesis of novel 4,5-diaryl functionalized 3,4-dihydropyrimidine-2(1H)-thiones via a non-biginelli-type approach and evaluation of their in vitro anticancer activity. Org. Biomol. Chem. 2014, 12, 3427–3440. [Google Scholar] [CrossRef] [PubMed]
- Nagarajaiah, H.; Mukhopadhyay, A.; dan Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Yoshida, N.; Kasuya, N.; Haga, N.; Fukuda, K. Brand-new biomass-based vinyl polymers from 5-hydroxymethylfurfural. Polym. J. 2008, 40, 1164–1169. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, S.; Li, T. p-Toluenesulfonic Acid-catalyzed efficient synthesis of dihydropyrimidines: Improved high yielding protocol for the biginelli reaction. Synth. Commun. 2002, 32, 1847–1851. [Google Scholar] [CrossRef]
- Matache, M.; Dobrota, C.; Bogdan, N.D.; Dumitru, I.; Ruta, L.L.; Paraschivescu, C.C.; Farcasanu, I.C.; Baciu, I.; Funeriu, D.P. Synthesis of fused dihydro-pyrimido [4, 3-d] coumarins using Biginelli multicomponent reaction as key step. Tetrahedron 2009, 65, 5949–5957. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds, Tables of Spectral Data, 4th Completely Revised ed.; Springer: Berlin, Germany, 2009; pp. 70, 134, 138. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Brooks/Cole, Cengage Learning: Belmont, CA, USA, 2009; pp. 151, 178, 293. [Google Scholar]
- Breitmaier, E. Structure Elucidation by NMR in Organic Chemistry: A Practical Guide, 3th Revised ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2002; pp. 11–12. [Google Scholar]
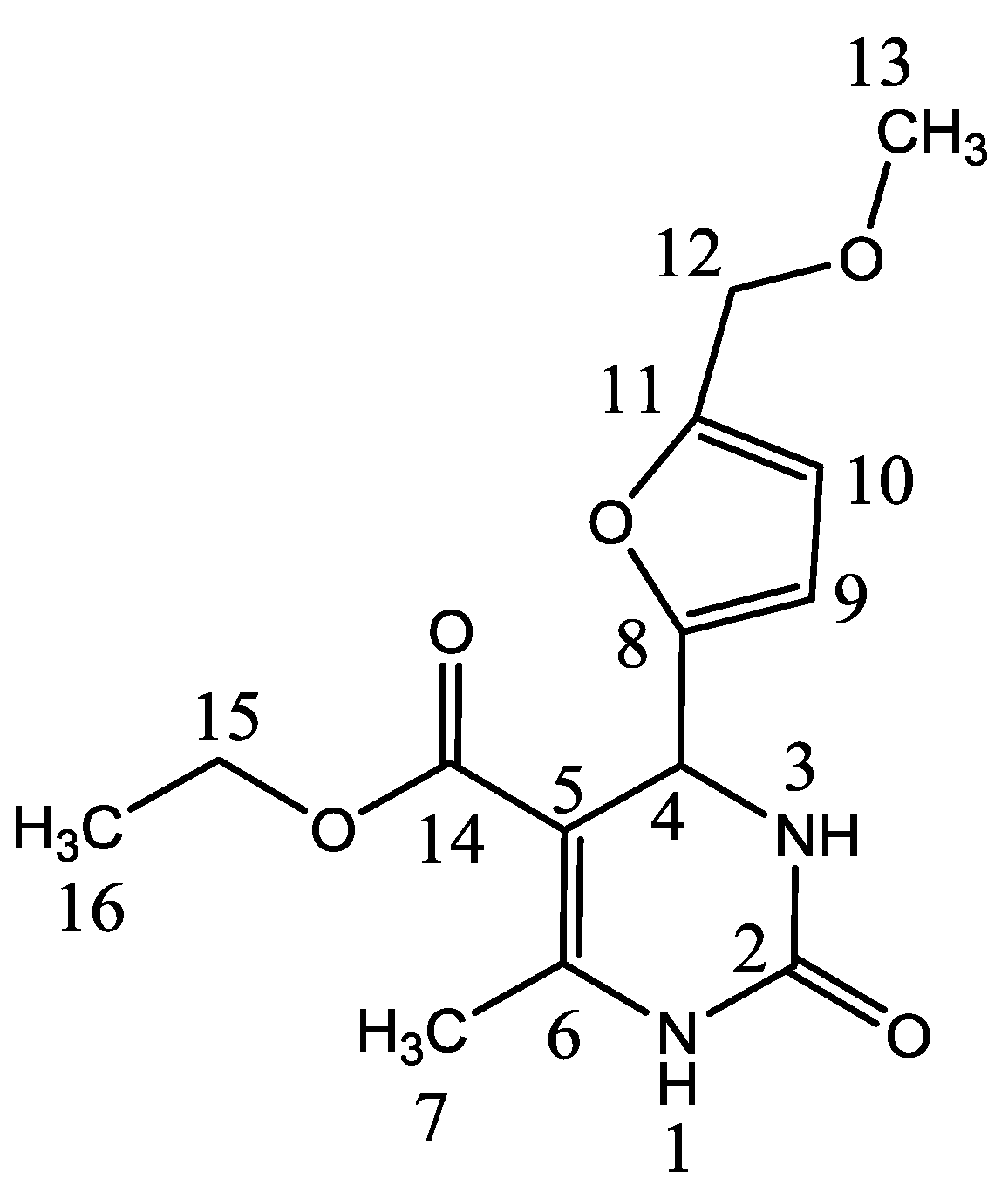
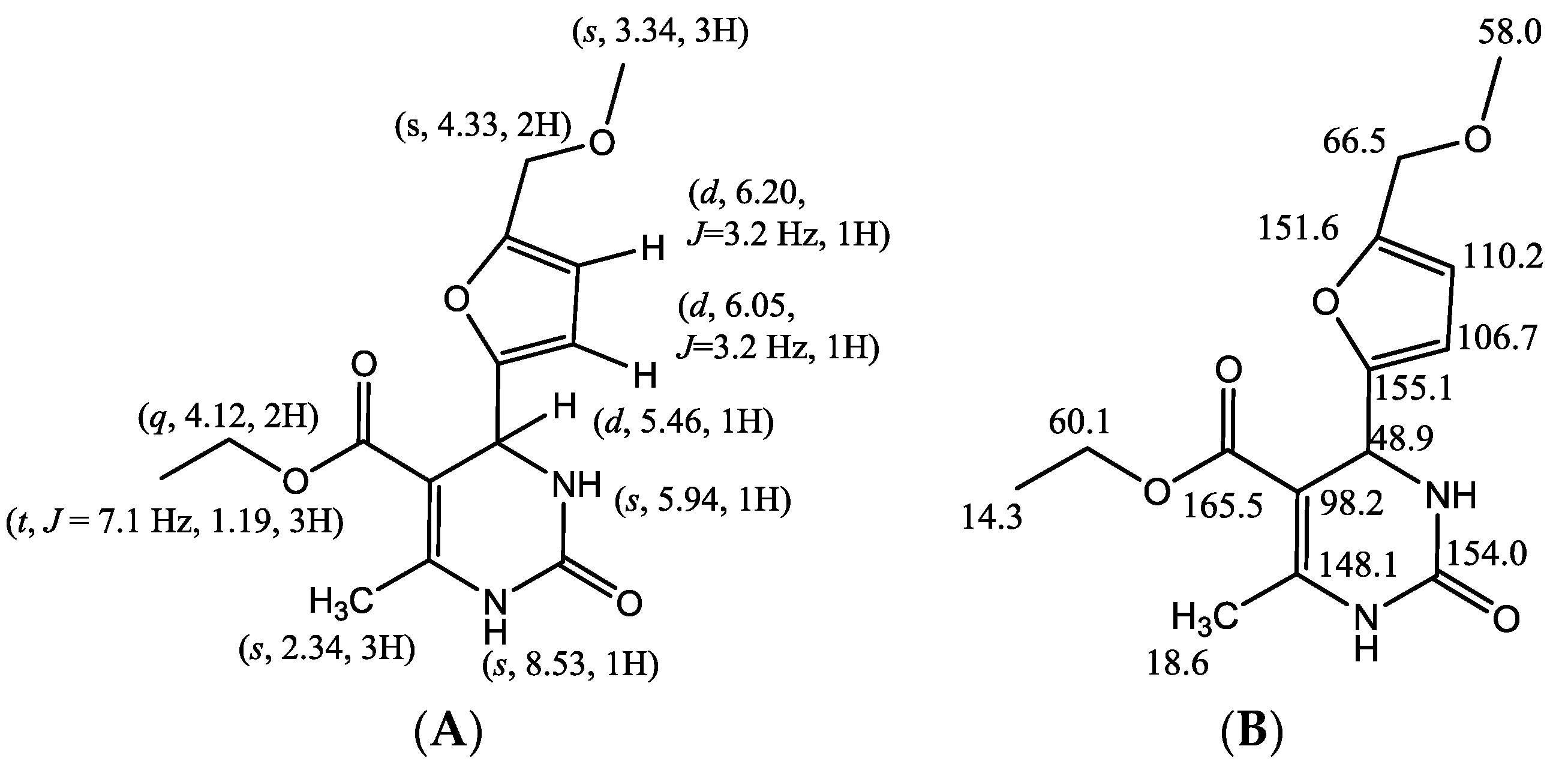
| No. Atom | δH (mult, J Hz) | δC (ppm) | HMBC |
|---|---|---|---|
| 1 | 8.53 (s, 1H) | -- | C-5, C-6, C-7, C-14 |
| 2 | 154.0 | ||
| 3 | 5.94 (s, 1H) | -- | C-4, C-5 |
| 4 | 5.46 (d, J = 3.0 Hz, 1H) | 48.9 | C-2, C-5, C-6, C-8, C-9, C-14 |
| 5 | 98.2 | ||
| 6 | 148.1 | ||
| 7 | 2.35 (s, 3H) | 18.6 | C-6, C-4, C-5, C-14 |
| 8 | 155.1 | ||
| 9 | 6.05 (d, J = 3.2 Hz, 1H) | 106.7 | C-8, C-10, C-11 |
| 10 | 6.20 (d, J = 3.2 Hz, 1H) | 110.2 | C-9, C-8, C-11 |
| 11 | 151.6 | ||
| 12 | 4.33 (s, 2H) | 66.5 | C-10, C-11, C-13 |
| 13 | 3.34 (s, 3H) | 58.0 | C-12 |
| 14 | 165.5 | ||
| 15 | 4.12 (q, 2H) | 60.1 | C-16 |
| 16 | 1.19 (t, J = 7.1 Hz, 3H) | 14.3 | C-15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwito, H.; Zulqaida, S.; Ul Haq, K.; Novi Kristanti, A.; Indriani, I. Ethyl 4-[5-(methoxymethyl)furan-2-yl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2017, 2017, M954. https://doi.org/10.3390/M954
Suwito H, Zulqaida S, Ul Haq K, Novi Kristanti A, Indriani I. Ethyl 4-[5-(methoxymethyl)furan-2-yl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank. 2017; 2017(3):M954. https://doi.org/10.3390/M954
Chicago/Turabian StyleSuwito, Hery, Salma Zulqaida, Kautsar Ul Haq, Alfinda Novi Kristanti, and Indriani Indriani. 2017. "Ethyl 4-[5-(methoxymethyl)furan-2-yl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate" Molbank 2017, no. 3: M954. https://doi.org/10.3390/M954
APA StyleSuwito, H., Zulqaida, S., Ul Haq, K., Novi Kristanti, A., & Indriani, I. (2017). Ethyl 4-[5-(methoxymethyl)furan-2-yl]-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank, 2017(3), M954. https://doi.org/10.3390/M954




