Abstract
The title compound, (E)-3′,6′-bis(diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one, was synthesized in 92% isolated yield using microwave-assisted organic synthesis. This new rhodamine derivative was fully characterized by 1H-NMR, 13C-NMR, FTIR and high resolution MS.
1. Introduction
The rhodamines are a highly fluorescent class of compounds used in many different fields of research, from fluorescent chemical sensors to biological stains and markers for cellular drug resistance [1,2,3,4,5]. Fluorescent molecular sensors have become an important and widely used tool for the real-time monitoring of metal ion concentrations in biological samples. Most such sensors reported so far have a transition metal chelating site linked to a fluorophore, and the metal binding causes the change in fluorescence intensity [6,7,8,9,10,11,12,13,14]. Selective detection of these metals has potential applications in many fields including chemistry, medicine, biology, and the environment [6,7,8,9]. Rhodamine B derivatives have received a great deal of attention as chemosensors due to their useful properties such as a high absorption coefficient, and high fluorescent quantum yield for excitation and emission wavelength within the visible region. Moreover, rhodamine B derivatives can undergo equilibrium between their spirocyclic and ring-open forms, which have completely different fluorescent properties.
In this note, we report the preparation of the rhodamine derivative L- that is, (E)-3′,6′-bis(diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one. Herein, we report the use of microwave-assisted organic synthesis of rhodamine derivative (L) in yields ranging from 74% to 92%. The significant reduction in solvent and reaction time as well as the derivative’s better purity offer privileges over other methods where complex chromatographic techniques are required for the purification of the target compounds. The synthesis of this compound was motivated by the excellent properties of rhodamine B, which is the backbone for the target compound (L). The target compound is a very fluorescent material, and we anticipate that it can be used for the development of chemical sensors. This new rhodamine derivative was fully characterized by melting point, 1H-NMR, 13C-NMR, FTIR and high resolution MS (HRMS).
2. Results and Discussion
The synthesis of the compound (E)-3′,6′-bis(diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one (L) was achieved in two steps, as presented in Scheme 1, which was performed by a condensation reaction of 2-amino-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one (2) [15] with 2-methoxy-1-naphthaldehyde. The reaction was carried out in ethanol at different temperatures and with different reaction times. The desired product L was obtained in 92% yield at a reaction temperature of 100 °C. The structure of compound L was confirmed by 1H-NMR, 13C-NMR, FTIR and HRMS, and all data are in accordance with the proposed structure.
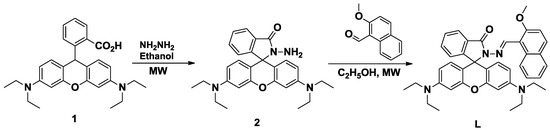
Scheme 1.
Synthesis of (E)-3′,6′-bis(diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one (L).
3. Materials and Methods
3.1. General Information
Reagents and solvents were purchased as reagent-grade and used without further purification unless otherwise stated. 1H- and 13C-NMR spectra were recorded on a Bruker Avance II 400 spectrometer (Bruker Biospin, Karlsruhe, Germany) in CDCl3. Data for 1H-NMR are reported as follows: chemical shifts (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), integration and coupling constant (Hz). Data for 13C-NMR are reported in terms of chemical shift. NMR spectra were analyzed using MestReNova software (version 10, Mestrela research, Feliciano Barrera, Spain). The IR spectrum was obtained using an FTIR spectrometer (Shimadzu, IRAffinity-1S, Columbia, MD, USA), and reported in terms of frequency of absorption (cm−1). Microwave-assisted reactions were carried out in a single mode Biotage Initiator 2.0.
3.2. Synthesis of 2-Amino-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one (2)
Using microwave heating protocols, a mixture of rhodamine B (0.12 g, 0.25 mmol), an excess of hydrazine hydrate (80%) (0.3 mL), and ethanol (3 mL) was placed in a 10-mL reaction vial [15]. The resulting mixture was stirred to make it homogenous and it was placed in the cavity of Biotage microwave reactor irradiated for 10 min at 100 °C. After cooling to room temperature, the resulting solid was filtered and washed three times with water. After drying, the 2-amino-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one (2) was isolated to give the yields (85%). Melting point: 176–177 °C; 1H-NMR (CDCl3), δ (ppm): 1.14 (12H, t, J = 7.2 Hz, NCH2CH3), 3.31 (8H, q, J = 7.2 Hz, NCH2CH3), 3.64 (2H, broad s, NH2), 6.31 (2H, dd, J = 8.8 and J = 2.4 Hz, H-2, 7), 6.44 (2H, d, J = 2.4 Hz, H-4, 5), 6.48 (2H, d, J = 8.8 Hz, H-1, 8), 7.12–7.14 (1H, m, Ar-H), 7.46–7.50 (2H, m, Ar-H), 7.95–7.97 (1H, m, Ar-H). 13C-NMR (CDCl3), δ (ppm): 12.7 (NCH2CH3), 44.4 (NCH2CH3), 66.0 (spiro carbon), 77.1, 98.0, 104.6, 108.0, 108.1, 122.9, 123.8, 128.1, 130.0, 132.5, 148.9, 151.6, 153.9, 166.
3.3. Synthesis of (E)-3′,6′-bis(Diethylamine)-2-[(2-methoxynaphthalen-1-yl)methyleneamino]spiro[isoindoline-1,9′-xanthen]-3-one (L)
Using microwave heating protocols, a mixture of 2 (105 mg, 0.230 mmol), 2-methoxy-1-naphthaldehyde (41 mg, 0.220 mmol), and ethanol (2 mL) was placed in a 10-mL reaction vial. The resulting mixture was stirred to make it homogeneous and then placed in the cavity of a biotage microwave reactor (MW) with a power of 180 W. The closed reaction vessel was run under pressure and the reaction was irradiated according to the parameters described in Table 1. This reaction was performed safely at a maximum temperature of 100 °C. However, reactions can safely be performed at pressures up to 20 bar and temperatures ranging from 40 °C to 250 °C. After cooling to room temperature, the resulting solid was filtered and washed three times with cold ethanol. After drying, the compound (L) was isolated to give the yields presented in Table 1. Melting point: 244–246 °C; 1H-NMR (CDCl3), δ (ppm): 1.14 (12H, t, J = 6.9 Hz, NCH2CH3), 3.31 (8H, q, J = 6.9 Hz, NCH2CH3), 3.82 (3H, s, OCH3), 6.28 (2H, dd, J = 8.8 Hz, 2.6 Hz), 6.44 (2H, d, 2.2 Hz), 6.63 (2H, d, 8.8 Hz), 7.09 (1H, d, J = 4.9 Hz), 7.12 (1H, d, J = 8.4 Hz), 7.15–7.27 (2H, m, H-Ar), 7.48–7.51 (2H, m, H-Ar), 7.63 (1H, d, 7.7 Hz, H-Ar), 7.71(1H, d, J = 8.0, H-Ar), 7.74 (1H, d, J = 8.4 Hz, H-Ar), 8.77 (1H, d, J = 7.4 Hz, H-Ar), 9.63 (1H, s, N=C-H); 13C-NMR (CDCl3), δ (ppm): 12.7 (NCH2CH3), 44.3 (NCH2CH3), 56.7, 66.3 (spiro carbon), 79.9, 104.6, 106.5, 107.9, 108.1, 112.9, 116. 8, 123.2, 124.0, 126.7, 127, 128.1, 129.2, 130.3, 131.9, 133.1, 137.6, 147.6 (N=C-H), 148.8, 151.7, 153.4, 157.8, 164.6; HRMS (ESI) m/z: [M + H]+ Calcd. for: C40H40N4O3 625.3173; Found: 625.3176.

Table 1.
Step 2: experiments to prepare the title compound (L) using different heating methods.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-8599/2017/3/M955/s1, Figure S1: 1H-NMR spectrum of L, Figure S2: 13C-NMR spectrum of L, Figure S3: HRMS (ESI) spectrum of L and S4: FTIR spectrum of L.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Acknowledgments
This work was supported by the Department of chemistry, Morgan State University.
Author Contributions
F.A. conceived and designed the experiments; P.P. performed the microwave reaction experiments; A.W. contributed reagents/materials/analysis tools; F.A. analyzed and confirmed the data analysis and wrote the paper.
Conflicts of interest
The authors declare no conflict of interest.
References
- Lohar, S.; Banerjee, A.; Sahana, A.; Banik, A.; Mukhopadhyay, S.; Das, D. A rhodamine-naphthalene conjugate as a FRET based sensor for Cr3+ and Fe3+ with cell staining application. Anal. Methods 2013, 5, 442–445. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sasaoka, N.; Tsuchida, T.; Fujiware, T.; Nagao, S.; Ohmoto, T. Fluorescent dye rhodamine 6G as a molecular probe to study drug resistance of C6 rat glioma cells. J. Neuro-Oncol. 1992, 13, 217–222. [Google Scholar] [CrossRef]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.; Franz, K. A cell-permeable fluorescent prochelator responds to hydrogen peroxide and metal ions by decreasing fluorescence. Inorg. Chim. Acta 2012, 380, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Aydin, Z.; Zhang, Y.; Liu, Z.; Guo, M. A turn-on fluorescent sensor for imaging labile Fe3+ in live neuronal cells at subcellular resolution. ChemBioChem 2012, 13, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, P.; Yan, W.; He, C.; Xiong, L.; Li, F.; duan, C. A bright water-compatible sugar-rhodamine fluorescence sensor for selective detection oh Hg2+ in natural water and living cells. J. Environ. Monit. 2009, 11, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hue, F.J.; Su, J.; Sun, Y.Q.; Yin, C.X.; Tong, H.B.; Nie, Z.X. A rhodamine-based dual chemosensor for the visual detection of copper and the ratiometric fluorescent detection of vanadium. Dyes Pigment. 2010, 86, 50–55. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Qian, X. A rhodamine-based Hg2+ sensor with high selectivity and sensitivity in aqueous solution: A NS2-containing receptor. J. Org. Chem. 2009, 74, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, A.J.; Abebe, F.A.; Venter, A.; Sinn, E. Rhodamine based turn-on sensors for Cr3+ and Ni2+: Detecting CN− via the metal displacement approach of sensor-Cr3+ complex. J. Fluoresc. 2016, 26, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Weerasinghe, A.; Abebe, F.; Sinn, E. Rhodamine based turn-on dual sensor for Fe3+ and Cu2+. Tetrahedron Lett. 2011, 52, 5648–5651. [Google Scholar] [CrossRef]
- Wang, H.H.; Xue, L.; Yu, C.L.; Qian, Y.Y.; Jiang, H. Rhodamine-based fluorescent sensor for mercury in buffer solution and living cells. Dyes Pigment. 2011, 91, 350–355. [Google Scholar] [CrossRef]
- Abebe, F.; Sinn, E. Fluorescein-based fluorescent and colorimetric chemosensors for copper in aqueous media. Tetrahedron Lett. 2011, 52, 5234–5237. [Google Scholar] [CrossRef]
- Abebe, F.; Eribal, C.; Ramakrishna, G.; Sinn, E.A. “Turn-on” fluorescent sensor for the selective detection of Cobalt and Nickel ions in aqueous media. Tetrahedron Lett. 2011, 52, 5554–5558. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanism, and applications in living cells. J. Am. Chem. Soc. 2009, 132, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.; Silva, A.; Cunha-Silvs, L.; Castro, B.; Gameiro, P.; Rangel, M. Discrimination of fluorescence light-up effects induced by PH and metal ion chelation on a spirocyclic derivative of rhodamine B. Dalton Trans. 2013, 42, 6110–6118. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).