Abstract
In an attempt to enhance cytotoxic activity of pyrazolo[3,4-d]pyrimidine core, we synthesized (3,5-dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone (4) by reacting 4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)benzohydrazide (3) with acetylacetone. Antiproliferative activity of this compound was screened against breast (MCF-7), colon (HCT-116), and liver (HEPG-2) cancer cell lines. The tested compound exhibited cytotoxic activity with IC50 = 5.00–32.52 μM. Moreover, inhibitory activity of this compound was evaluated against the epidermal growth factor receptor (EGFR), the fibroblast growth factor receptor (FGFR), the insulin receptor (IR), and the vascular endothelial growth factor receptor (VEGFR). This target compound showed potent inhibitory activity, especially against FGFR with IC50 = 5.18 μM.
1. Introduction
Protein kinases play an important role in cell proliferation, differentiation, migration, metabolism, and apoptosis [1,2]. Dysregulation of protein kinases occurs in a variety of diseases including cancer [3,4]. Overexpression of protein kinase in tumor cells can be blocked by selective kinase inhibitors, so these inhibitors are considered a promising approach for treatment of cancer [5,6,7,8].
Pyrazolo[3,4-d]pyrimidine derivatives attract great interest because of their diverse biological and pharmacological properties. Among these properties, their anticancer effects have been extensively evaluated [9,10,11,12].
The cytotoxic effect of this class of compounds is attributed to different mechanisms. They have been reported as acting as cyclin dependent kinase (CDK) inhibitors [13,14,15], glycogen synthase kinase (GSK) inhibitors [16,17], epidermal growth factor receptor (EGFR) inhibitors [18], and dual src/Ab1 kinase inhibitors [19].
A new series of 2-(3,6-dimethyl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yloxy)- N-(4-substitutedbenzylidene)acetohydrazides, specifically compound 1 (Figure 1), displayed cytotoxic activity against breast carcinoma (MCF-7), non-small cell lung cancer (A549), and human colorectal adenocarcinoma (HT-29) cell lines, and proved to be inhibitors of EGFR protein kinase [20]. El Hamid et al. synthesized and evaluated a new set of 1-aryl-4-benzylidenehydrazinyl-3-methylsulphanyl-pyrazolo[3,4-d]pyrimidines as anti-breast cancer agents. Compound 2 was the most active compound in this search with an IC50 equal to 7.5 nM [21]. In addition, the literature survey revealed that the pyrazole moiety represents an important pharmacophore in several anticancer active agents [22,23,24,25,26,27,28]. All these facts encouraged us to hybridize the pyrazolo[3,4-d]pyrimidine scaffold with the pyrazole nucleus in a trial to obtain a promising new anticancer active agent.
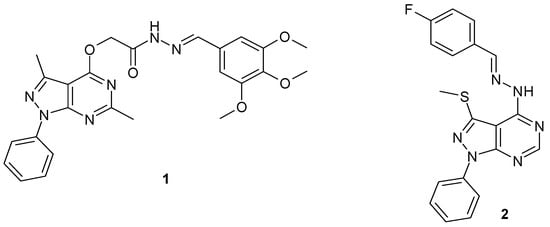
Figure 1.
Chemical structures of some reported pyrazolo[3,4-d]pyrimidine derivatives as anticancer agents.
2. Results and Discussion
2.1. Chemistry
(3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone (4) was prepared by refluxing 4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)benzohydrazide (3) [29] with acetylacetone in acetic acid for 10 h, as shown in Scheme 1.
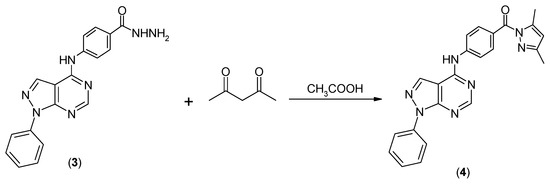
Scheme 1.
Synthetic pathway for target compound 4.
The structure of the target compound 4 was elucidated by IR, 1H-, and 13C-NMR, MS, and elemental analysis. All data are in accordance with the assumed structure. The IR spectrum of this compound revealed the presence of a NH group at 3232 cm−1 and a C=O group at 1697 cm−1.
The 1H-NMR spectrum showed two singlet signals at δ 2.20 and 2.56 ppm corresponding to the two methyl protons in addition to a singlet signal at δ 6.28 ppm, corresponding to the pyrazole H-4proton. Inspection of the 13C-NMR spectrum indicated an appearance of two methyl peaks at δ 13.90 and 14.27 ppm and pyrazole C4 at δ 111.53 ppm. Finally, the mass spectrum of 4 demonstrated a molecular ion peak at m/z 409 (M+.) and a base peak at m/z 314.
2.2. Pharmacological Screening
The newly synthesized compound was screened in vitro for its anticancer activity against three cancer cell lines—breast (MCF-7), liver (HEPG-2), and colon (HCT-116) cancer cell lines. The results were expressed in terms of IC50 values (the concentration that resulted in a 50% inhibition of cell viability) where the well-known anticancer agents doxorubicin was used as a positive control (Table 1).

Table 1.
Cytotoxic activity of compound 4 and doxorubicin on three cancer cell lines—MCF-7, HEPG-2, and HCT-116.
The tested compound showed a marked antitumor activity against all the tested cell lines with IC50 values of 5.00–32.52 μM. Moreover, this compound was more potent (IC50 = 5.00 μM) than doxorubicin (IC50 = 5.66 μM) against the liver cancer cell line (HEPG-2).
Furthermore, this compound was evaluated against different protein kinases such as the epidermal growth factor receptor (EGFR), the fibroblast growth factor receptor (FGFR), the insulin receptor (IR), and the vascular endothelial growth factor receptor (VEGFR) in an attempt to explore the mechanism of action of this compound. The obtained results demonstrated that this compound exhibited good inhibitory activity against all used protein kinases with IC50 values of 5.18–27.89 μM (Table 2).

Table 2.
Kinase inhibitory activity of compound 4.
3. Materials and Methods
3.1. Chemistry
Melting points was determined on a Thomas–Hoover capillary apparatus and are uncorrected. Infrared (IR) spectrum was recorded as films on NaCl plates using a Nicolet 550 Series II Magna FT-IR spectrometer (Shimadzu, Kyoto, Japan). 1H-NMR and 13C-NMR spectra were measured on a BrukerAvance III 400 MHz for 1H and 100 MHz for 13C (Bruker AG, Fällanden, Switzerland) with a BBFO Smart Probe and a Bruker 400 AEON Nitrogen-Free Magnet, Faculty of Pharmacy, Beni-Suef University, Egypt in DMSO-d6 with TMS as the internal standard, where J (coupling constant) values are estimated in Hertz (Hz), and chemical shifts were recorded in ppm δ scale. Mass spectrum (MS) was recorded on a Hewlett Packard 5988 spectrometer (Hewlett-Packard Co., Palo Alto, CA, USA). Microanalyses for C, H, and N were carried out on a Perkin–Elmer 2400 analyzer (Perkin-Elmer, Norwalk, CT, USA) at the Micro analytical unit of Cairo University, Egypt All other reagents, purchased from the Aldrich Chemical Company (Milwaukee, WI, USA), were used without further purification. 4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)benzohydrazide (3) was prepared according to a literature procedure [29].
(3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone) (4)
A mixture of 4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)benzohydrazide (3, 3.59 g, 10 mmol) and acetylacetone (1 g, 10 mmol) in acetic acid (10 mL) was heated under reflux for 10 h. After cooling, the reaction mixture was poured onto ice water. The colorless powder obtained was crystallized from ethanol to yield compound 4. Mp 180–182 °C; yield: 56%; IR (cm−1): 3232 (NH); 3070 (CH aromatic); 2954 (CH aliphatic); 1697 (C=O); 1608 (C=N); 1H-NMR (DMSO-d6) δ ppm: 2.20 (s, 3H, CH3), 2.56 (s, 3H, CH3), 6.28 (s, 1H, pyrazole H-4 7.37–7.41 (m, 1H, phenyl H-4), 7.57–7.61 (m, 2H, phenyl H-3, H-5), 8.01 (d, J = 8.8 Hz, 2H, aminophenyl H-2, H-6), 8.09 (d, J = 8.8 Hz, 2H, aminophenyl H-3, H-5), 8.21 (s, 1H, pyrazole CH), 8.23 (s, 1H, pyrimidine CH), 8.67 (d, J = 12 Hz, 2H, phenyl H-2, H-6), 10.55 (s, 1H, NH, D2O exchangeable); 13C-NMR (DMSO-d6) δ: 13.90, 14.27, 103.35, 111.53, 119.80, 121.45, 127.06, 127.53, 129.70, 132.74, 134.09, 138.95, 143.48, 144.85, 151.92, 153.45, 154.63, 156.34, 167.43; EIMS (m/z) 409 (M+., 28%), 314 (100%). Anal.Calcd for C23H19N7O: C, 67.47; H, 4.68; N, 23.95. Found: C, 67.53; H, 4.42; N, 24.01.
3.2. Pharmacological Studies
3.2.1. Cell Viability Analysis
The mammary gland breast cancer cell line (MCF-7), the human hepatocellular carcinoma cell line (HEPG-2), and the colon carcinoma cell line (HCT-116) were obtained from the American Type Culture Collection (ATCC). Doxorubicin (positive control) and all chemicals used in this study are of high analytical grade and were obtained from either Sigma-Aldrich or Biorad. The different cell lines mentioned above were used to determine the inhibitory effects of the tested compounds on cell growth using the SulphoRhodamine-B (SRB) assay using the method of Skehan et al. [30]. Cells were plated in 96 multi-well plates for 24 h before treatment with the compounds to allow attachment of the cells to the wall of the plate. Different concentrations of the tested compounds (0, 6.25, 12.5, 25, 50, and 100 µg/mL) were added to the cell monolayer. Triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the compounds for 48 h at 37 °C and in an atmosphere of 5% CO2; cells were fixed, washed, and stained with Sulforhodamine B stain. Excess stain was washed with acetic acid and attached stain was recovered with a Tris EDTA buffer. Color intensity was measured in an ELISA reader and the relation between surviving fraction and drug concentration was plotted and IC50 (the concentration required for 50% inhibition of cell viability) was determined for each compound by Sigma plot software (Sigma Plot 11.0 software, Systat Software Inc.; San Jose, California).
3.2.2. Kinases Inhibitory Activity
Kinase activity was determined using Kinase-Glo Plus luminescence kinase assay kit according to the previously reported method [31].
Supplementary Materials
The following are available online at www.mdpi.com/1422-8599/2016/4/M915, Figure S1: spectrum of compound 4, Figure S2: 13C-NMR spectrum of compound 4, Figure S3: IR spectrum of compound 4.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Rania B. Bakr is grateful to all members of the Pharmaceutical Organic Chemistry, Faculty of pharmacy, Beni-suef university for all help and supports during progress of research.
Author Contributions
R.B.B. conceived, designed, and performed the experiments, analyzed the data, and wrote the paper. A.B.M. performed the anticancer screening and kinase inhibitory activity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase-role and significance in cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, I.N. Mechanisms of activation of receptor tyrosine kinases: Monomers or dimers. Cells 2014, 3, 304–330. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem. Biophys. Res. Commun. 2004, 319, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Na, I.-K.; le Coutre, P. Emerging Role of Tyrosine Kinases as Drugable Targets in Cancer. Biomark. Insights 2015, 10, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Sachsenmaier, C. Targeting protein kinases for tumor therapy. Oncol. Res. Treat. 2001, 24, 346–355. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Parrino, B.; Lopergolo, A.; Pennati, M.; Zaffaroni, N.; Cirrincione, G. Synthesis and Antitumor Activity of 3-(2-Phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-Phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem 2011, 6, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Barraja, P.; Montalbano, A.; Parrino, B.; Spanò, V.; Lopergolo, A.; Sbarra, S.; Doldi, V.; Zaffaroni, N. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]pyridines, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014, 21, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; De Cesare, M. Novel 1H-pyrrolo[2,3-b]pyridine derivative nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Alqasoumi, S.I.; Alafeefy, A.M.; Aboulmagd, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Traxler, P.; Bold, G.; Frei, J.; Lang, M.; Lydon, N.; Mett, H.; Buchdunger, E.; Meyer, T.; Mueller, M.; Furet, P. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J. Med. Chem. 1997, 40, 3601–3616. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.B.; Abdelall, E.K.; Abdel-Hamid, M.K.; Kandeel, M.M. Design and synthesis of new EGFR-tyrosine kinase inhibitors containing pyrazolo[3,4-d]pyrimidine cores as anticancer agents. Bull. Pharm. Sci. Assiut. Univ. 2012, 35, 1–16. [Google Scholar]
- Abdellatif, K.R.; Abdelall, E.K.; Abdelgawad, M.A.; Ahmed, R.R.; Bakr, R.B. Synthesis, docking study and antitumor evaluation of certain newly synthesized pyrazolo[3,4-d]pyrimidine derivatives. Organ. Chem. Indian J. 2014, 10, 157–167. [Google Scholar]
- Kim, D.C.; Lee, Y.R.; Yang, B.-S.; Shin, K.J.; Kim, D.J.; Chung, B.Y.; Yoo, K.H. Synthesis and biological evaluations of pyrazolo[3,4-d]pyrimidines as cyclin-dependent kinase 2 inhibitors. Eur. J. Med. Chem. 2003, 38, 525–532. [Google Scholar] [CrossRef]
- Fernandez, M.; Tundidor-Camba, A.; Caballero, J. Modeling of cyclin-dependent kinase inhibition by 1H-pyrazolo[3,4-d]pyrimidine derivatives using artificial neural network ensembles. J. Chem. Inf. Model. 2005, 45, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Havlícek, L.; McNae, I.W.; Walkinshaw, M.D.; Voller, J.; Šturc, A.N.; Navrátilová, J.; Kuzma, M.; Mistrík, M.; Bártek, J. Pyrazolo[4,3-d]pyrimidine bioisostere of roscovitine: Evaluation of a novel selective inhibitor of cyclin-dependent kinases with antiproliferative activity. J. Med. Chem. 2011, 54, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Peat, A.J.; Boucheron, J.A.; Dickerson, S.H.; Garrido, D.; Mills, W.; Peckham, J.; Preugschat, F.; Smalley, T.; Schweiker, S.L.; Wilson, J.R. Novel pyrazolopyrimidine derivatives as GSK-3 inhibitors. Bioorgan. Med. Chem. Lett. 2004, 14, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Peat, A.J.; Garrido, D.; Boucheron, J.A.; Schweiker, S.L.; Dickerson, S.H.; Wilson, J.R.; Wang, T.Y.; Thomson, S.A. Novel GSK-3 inhibitors with improved cellular activity. Bioorgan. Med. Chem. Lett. 2004, 14, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Ducray, R.; Ballard, P.; Barlaam, B.C.; Hickinson, M.D.; Kettle, J.G.; Ogilvie, D.J.; Trigwell, C.B. Novel 3-alkoxy-1H-pyrazolo[3,4-d]pyrimidines as EGFR and erbB2 receptor tyrosine kinase inhibitors. Bioorgan. Med. Chem. Lett. 2008, 18, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Mosti, L.; Maga, G.; Crespan, E.; Carraro, F.; Manetti, F.; Tintori, C. Synthesis, biological evaluation and docking studies of 4-amino substituted 1H-pyrazolo[3,4-d]pyrimidines. Eur. J. Med. Chem. 2008, 43, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Bakr, R.B.; Alkhoja, O.A.; Mohamed, W.R. Design, synthesis and antitumor activity of novel pyrazolo[3,4-d]pyrimidine derivatives as EGFR-TK inhibitors. Bioorgan. Chem. 2016, 66, 88–96. [Google Scholar] [CrossRef] [PubMed]
- El Hamid, M.K.A.; Mihovilovic, M.D.; El-Nassan, H.B. Synthesis of novel pyrazolo[3,4-d]pyrimidine derivatives as potential anti-breast cancer agents. Eur. J. Med. Chem. 2012, 57, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Draghici, C.; Missir, A.V. Synthesis of new pyrazole derivatives and their anticancer evaluation. Eur. J. Med. Chem. 2010, 45, 4914–4919. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Rostom, S.A.; Al-Saadi, M.S. Synthesis and biological evaluation of some new substituted fused pyrazole ring systems as possible anticancer and antimicrobial agents. Science (JKAU: Sci.) 2010, 22, 177–191. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Martorana, A.; Gia, O.; DallaVia, L.; Cirrincione, G. 3,5-Bis(3′-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorgan. Med. Chem. Lett. 2007, 17, 6134–6137. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Salvador, A.; Brun, P.; Vedaldi, D.; Diana, P.; Cirrincione, G.; Barraja, P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015, 102, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Spanò, V.; Giallombardo, D.; Diana, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of [1,2]oxazolo[5,4-e]indazoles as antitumour agents. Tetrahedron 2013, 69, 6474–6477. [Google Scholar] [CrossRef]
- Maggio, B.; Raimondi, M.V.; Raffa, D.; Plescia, F.; Cascioferro, S.; Cancemi, G.; Tolomeo, M.; Grimaudo, S.; Daidone, G. Synthesis and antiproliferative activity of 3-(2-chloroethyl)-5-methyl-6-phenyl-8-(trifluoromethyl)-5,6-dihydropyrazolo [3,4-f][1,2,3,5]tetrazepin-4-(3H)-one. Eur. J. Med. Chem. 2015, 96, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Maggio, B.; Raffa, D.; Raimondi, M.V.; Cascioferro, S.; Plescia, F.; Tolomeo, M.; Barbusca, E.; Cannizzo, G.; Mancuso, S.; Daidone, G. Synthesis and induction of G0–G1 phase arrest with apoptosis of 3,5-dimethyl-6-phenyl-8-(trifluoromethyl)-5,6-dihydropyrazolo[3,4-f][1,2,3,5]tetrazepin-4(3H)-one. Eur. J. Med. Chem. 2008, 43, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Abdelall, E.; BBakr, R.; Abdel-Hamid, M.; Kandeel, M. Enhancement to synthesize, design and dock of novel EGFR inhibitors containing pyrazolo[3,4-d]pyrimidine cores of expected anticancer activity. OCAIJ 2014, 10, 470–483. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Balzano, D.; Santaguida, S.; Musacchio, A.; Villa, F. A general framework for inhibitor resistance in protein kinases. Chem. Biol. 2011, 18, 966–975. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).