Abstract
Planar bis(1,2-dithiooxalato)nickelate(II), [Ni(dto)]2− reacts in aqueous solutions with lanthanide ions (Ln3+) to form pentanuclear, hetero-bimetallic complexes of the general composition [{Ln(H2O)n}2{Ni(dto)2}3]·xH2O. (n = 4 or 5; x = 9–12). The complex [{Ho(H2O)5}2{Ni(dto)2}3]·10H2O, Ho2Ni3, was synthesized and characterized by single crystal X-ray structure analysis and powder diffraction. The Ho2Ni3 complex crystallizes as monoclinic crystals in the space group P21/c. The channels and cavities, appearing in the crystal packing of the complex molecules, are occupied by a varying amount of non-coordinated water molecules.
1. Introduction
During the last decades, molecular hetero-bimetallic 3d–4f complexes with transition metals and trivalent lanthanide ions have gathered increasing interest due to their magnetic properties [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Such complexes are promising precursors for, e.g., catalysts, magnetic materials, luminescent materials and new molecular devices. Since lanthanide ions provide a large angular momentum and the f–f transition is less influenced by the ligand field compared to the spin-orbit coupling, magnetic behavior different from the transition metals has been observed [16]. When transition metals are bridged to the lanthanide ions by small bridging ligands 3d–4f complexes with interesting magnetic properties are formed, e.g., as single-molecule magnets [18]. The design and construction of extended hetero-nuclear 3d–4f complexes with a discrete multinuclear aggregation is still a challenge for coordination chemists. A series of pentanuclear, hetero-bimetallic 3d–4f complexes of the general composition [{Ln(H2O)n}2{Ni(dto)2}3]·xH2O (n = 4 or 5; x = 9–12) was first described by Trombé, Gleizes and Galy [19,20]. Dependent on the decreasing ionic radii of the lanthanide ions, two types of structures were observed: a monoclinic structure for the larger lanthanide ions (La–Dy) and a triclinic structure for the smaller lanthanide ions (Er, Yb). The analogue Ho2Ni3 complex is the borderline case between both structural forms and its structural type was unclear. Here we can report the structure of this complex [{Ho(H2O)5}2{Ni(dto)2}3]·10H2O, Ho2Ni3. The here reported complex reported here crystallizes in the monoclinic space group P21/c. The structural information of the Ho2Ni3 complex is new in this series to the best of our knowledge.
2. Results and Discussion
2.1. X-ray Structures
A single crystal of the complex Ho2Ni3 was measured by X-ray diffraction. Table S1 summarizes the crystallographic data and refinement parameters for this complex. The Ho2Ni3 complex crystallizes in the monoclinic space group P21/c. In the crystals, two different forms of enclosed water molecules can be observed: defined coordinated water at the lanthanide ion and non-stoichiometric amounts of inserted water in the cavities and between the layers of the complex molecules.
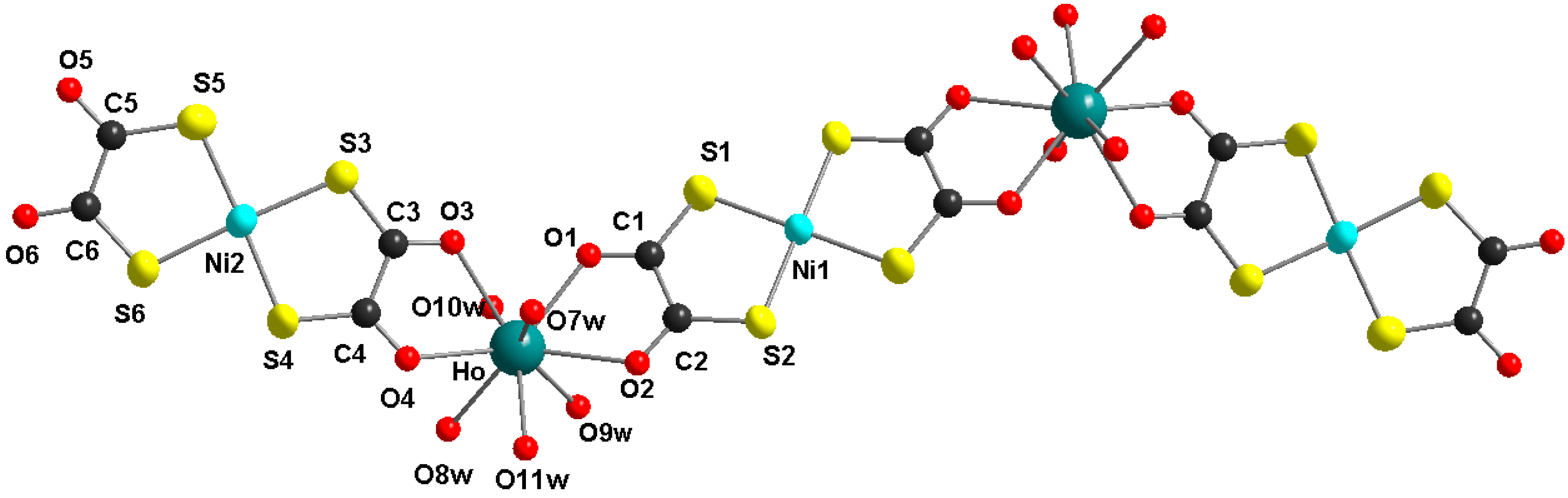
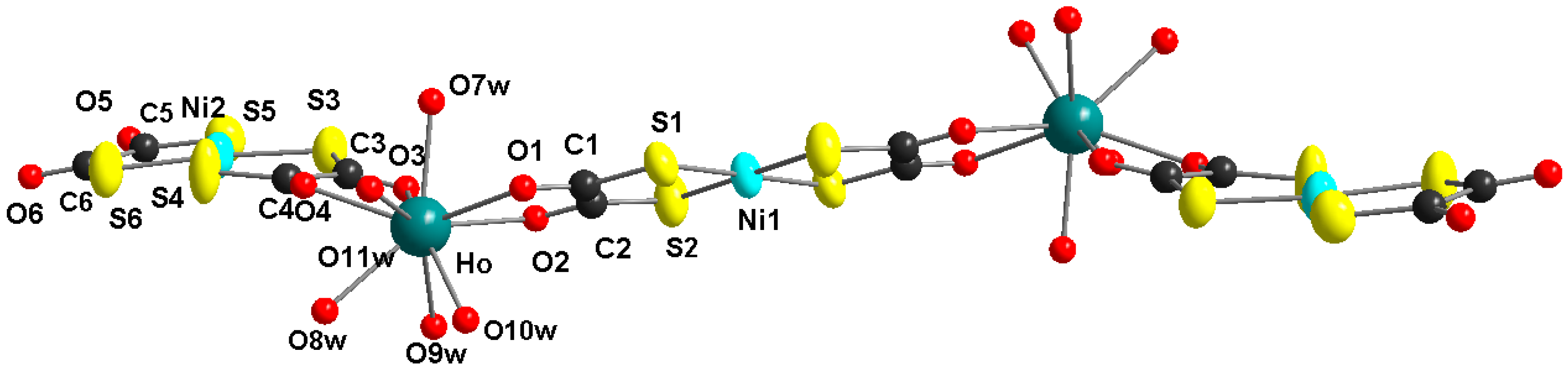
Figure 1 shows the molecular structure of the pentanuclear heterobimetallic Ho2Ni3 complex with the characteristic z-shape of this type of molecule [21,22,23,24]. The two holmium centers are bridged by a nearly planar bis(1,2-dithiooxalato)nickelate(II) unit. Two peripheric bis(1,2-dithiooxalato)nickelate(II) moieties are coordinated only bidentate in a non-bridging mode. The structure of the complex is close to planarity with a little twisting, due to the non-symmetric coordination geometry of the lanthanide ions (see Figure 2). The coordination spheres of the lanthanide ions are completed by five coordinated water ligands at each lanthanide ion, resulting in a coordination number of nine. The nine donor atoms in the coordination sphere of the lanthanide ion occupy the corners of a tricaped trigonal prism. The molecule is centrosymmetric with a crystallographic symmetry center at the Ni1 atom.
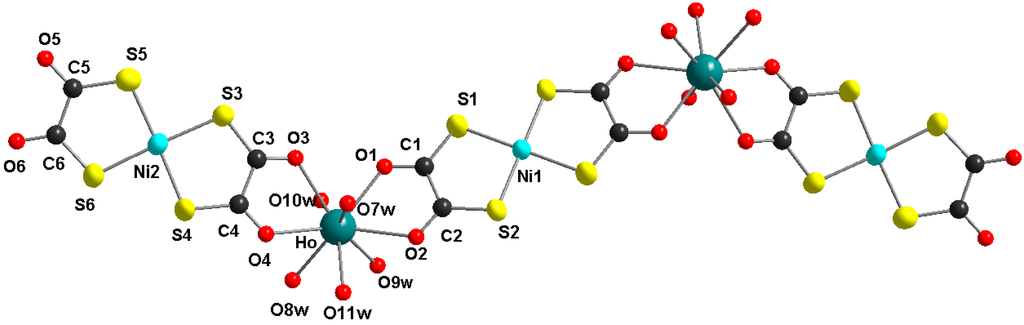
Figure 1.
Molecular structure of the Ho2Ni3 complex, view along the crystallographic a-axis (non-coordinated water molecules und hydrogen atoms are omitted for clarity).
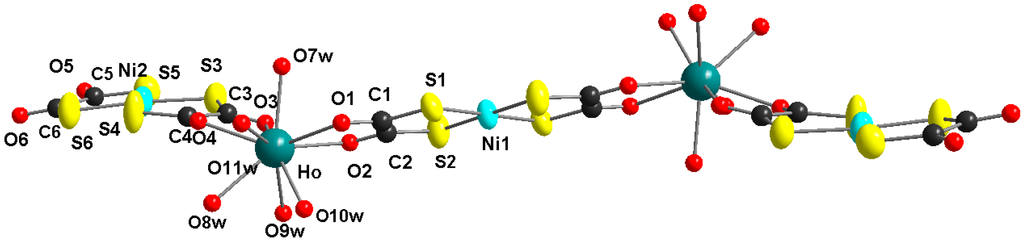
Figure 2.
Molecular structure of the Ho2Ni3 complex, view along the crystallographic b-axis (non-coordinated water molecules und hydrogen atoms are omitted for clarity).
The complex is almost isostructural to the already reported analogue Eu2Ni3, Nd2Ni3, Ce2Ni3, and Dy2Ni3 complexes [21,22,23,24]. The deviations in the structures are only due to small differences of the ion radii of the individual lanthanide ions. The crystal packing of the molecules contains channels and cavities, which are filled by non-coordinated water molecules. Whereas the coordinated water molecules are in fixed positions, the non-coordinated water molecules are strongly disordered. The slightly varying number of non-coordinated water molecules could be calculated (estimated) by thermoanalytical investigations and was determined with approximately nine to 12 inserted water molecules per formula unit [22]. This inserted water is very flexible and already partly released at laboratory conditions with dry air, making it difficult to determine their exact number. This also applies to the Ho2Ni3 complex in this series. Therefore, the crystal was completely embedded in perfluoropolyalkyl ether to protect it against decay during the X-ray crystal structure analysis. This effect is also responsible for the relatively high remaining electron density for this type of complex.
2.2. IR Spectroscopy
All these complexes show intense water signals around 3300 cm−1 in their IR absorption spectra (see Figure S2). Furthermore, very broad carbonyl vibrations (between 1600 cm−1 and 1400 cm−1) can be observed. This broadening results from the overlapping of the different types of coordinated and non-coordinated carbonyl groups present in the complex: non-coordinated terminal carbonyl groups of the peripheric 1,2-dithiooxalato ligands, which are found at approximately 1600 cm−1, comparable to the red form of the mononuclear K2[Ni(dto)2] complex [25], combine with the vibration of the carbonyl groups of the bridging dto ligands coordinated by the lanthanide ions. Also, the formation of intermolecular hydrogen bonds towards some of the carbonyl groups contributes to the broadening of the CO vibrations in the spectra. The resulting absorption bands of carbonyl vibrations are found at around 1495 cm−1. As already mentioned, the complexes of this series crystallize in two different crystal systems depending on the decreasing lanthanide radii, and this can also be recognized by IR spectroscopy. The comparison of vibration bands of v(C-C-S), v(S-C) and v(Ni-S) exhibits a shift of the absorptions in the range of 10 cm−1 if the crystal system changes from monoclinic to triclinic. These shifts are attributed to stronger bonding of the lanthanide ions and the dto ligands.
3. Experimental Section
3.1. General Methods, Analytical and Physical Measurements
All infrared spectra were recorded on a Perkin Elmer 16PC FT-IR-spectrometer in a range between 400 cm−1 and 4000 cm−1 using KBr pellets. The elemental analyses (C, H, S) were determined by a Vario EL III CHNS from elementar Analysensysteme GmbH (Hanau, Germany). The magnetic susceptibility was measured by a magnetic susceptibility balance MSB-Auto by Sherwood Scientific Ltd. at room temperature. XRD measurements were conducted with a Bruker AXS (Siemens) D5005 diffractometer using CuKα radiation (λ = 1.540598 Å) (see Figure S1).
X-ray structure of the presented complex was collected using a STOE Image Plate Diffraction System IPDS-2 at 210 K with graphite-monochromatized MoKα radiation. The reflection data were corrected by an absorption correction using the program X-Area [26]. The structures were solved with SHELXS-97 [27] using direct methods and refined with SHELXL-2014 [28]. The nonhydrogen atoms were refined anisotropically, with the exception of one non-coordinated oxygen atom of a crystal water molecule with an occupation factor of 0.25 in the structure. The hydrogen atoms of the water molecules could not be found. CCDC 1450593 contains the supplementary crystallographic data for this article and can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
3.2. Synthesis of [Ho2Ni3 (dto)6(H2O)10]·10H2O
The ligand potassium 1,2-dithiooxalate, K2dto, was synthesized by sulfhydrolysis of diphenyl oxalate according to the procedure of Matz and Mattes [29] modified by Wenzel et al. [30]. The complex Ho2Ni3 was prepared according to the general procedure described by Trombe et al. [20].
A solution of K2dto (1 mmol; 200.2 mg) in 5 mL of distilled H2O was added to a stirred solution of NiCl2·6 H2O (0.5 mmol; 119.7 mg) in 4 mL of distilled H2O. The resulting dark-violet solution was heated to 50 °C. A warm solution (50 °C) of HoCl3·xH2O (0.33 mmol) in 4 mL water was added drop wise to the stirred [Ni(dto2]2− - solution. The resulting reaction mixture was continuously stirred at 50 °C for additional 15 min, than slowly cooled down to room temperature. The dark-violet crystalline precipitate was filtered off, washed with a small amount of distilled water and dried at 80 °C.
K2dto: C2O2S2K2 (M = 198.35 g/mol). Elementary analysis (EA) measured (calculated): C 11.95 (12.11); S 32.08 (32.33) %. IR (KBr): 1530, 1514 (νC-O), 1113 (νC-C-S), 879 (νC-S) cm−1.
Ho2Ni3, 1: C12H22O23S12Ni3Ho2 (M = 1425.01 g/mol). EA meas. (calc.): C 10.05 (10.11); H 1.55 (1.56); S 26.58 (27.00) %. IR (KBr), Figure S2: 3166 (νOH), 1490 (νC-O), 1138 (νC-C-S), 990, (νC-S), 458 (νNi-S, δring) cm−1. μeff meas. (calc.): 14.63 (14.99) B.M.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4The following are available online at http://www.mdpi.com/1422-8599/2016/2/M895, Table S1: Crystallographic data and refinement parameters for the complex Ho2Ni3, Figure S1: Experimental X-ray powder patterns for Ho2Ni3 (1), Figure S2: IR absorption spectrum (KBr) of Ho2Ni3.
Acknowledgments
Christina Günter, University of Potsdam (Germany), Faculty of earth and environmental sciences for the powder diffraction measurements. Matthias Siebold, formerly University of Potsdam (Germany), Insitute of Chemistry for additional synthetic experiments.
Author Contributions
Jana König, Peter Strauch: writing of manuscript, analytical interpretation, literature search; Jana König: synthetic work; Alexandra Kelling, Uwe Schilde: X-ray crystallography.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| dto | 1,2-dithiooxalate |
| Ln | lanthanide |
References
- Sanz, J.L.; Ruiz, R.; Gleizes, A.; Lloret, F.; Faus, J.; Julve, M.; Borras-Almenar, J.J.; Journaux, Y. Crystal structure and magnetic properties of novel LnIIICuII (Ln = Gd, Dy, Ho) pentaanuclear complexes. Topology and ferromagnetic interaction in the LnIII–CuII pair. Inorg. Chem. 1996, 35, 7384–7393. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. A genuine example of a discrete bimetallic (Cu, Gd)–complex: structural determination and magnetic properties. Inorg. Chem. 1996, 35, 2400–2402. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. Experimental evidence of a ferromagnetic ground state (S=9/2) for a dinuclear Gd(III)–Ni(II) complex. Inorg. Chem. 1997, 36, 4284–4286. [Google Scholar] [CrossRef]
- Ramade, I.; Kahn, O.; Jeannnin, Y.; Robert, F. Design and magnetic properties of a magnetically isolated GdIIICuII pair. Crystal Structures of [Gd(hfa)3Cu(salen)], [Y(hfa)3Cu(salen)], [Gd(hfa)3Cu(salen)(Meim)], and [La(hfa)3H2OCu(salen)] [hfa = hexafluoroacetylacetonato, salen = N,N_-ethylenebis(salicylideneaminato), Meim = 1-Methylimidazole]. Inorg Chem. 1997, 36, 930–936. [Google Scholar] [CrossRef]
- Sanada, T.; Suzuki, T.; Kaizaki, S. Heterotrinuclear complexes containing d- and f-block elements: synthesis and structural characterization of novel lanthanide(III) nickel(II)-lanthanide(III) compounds bridged by oxamidate. J. Chem. Soc. Dalton Trans. 1998, 959–965. [Google Scholar] [CrossRef]
- Sanada, T.; Suzuki, T.; Yoshida, T.; Kaizaki, S. Heterodinuclear complexes containing d- and f-block elements: Synthesis, structural characterization, and metal–metal interactions of novel chromium(III)–lanthanide(III) compounds bridged by oxalate. Inorg. Chem. 1998, 37, 4712–4717. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Gross, M.; Schmalle, H.W.; Ferlay, S. Molecular chromium(III)-lanthanide(III) compounds (Ln = La, Ce, Pr, and Nd) with a polymeric, ladder-lype architecture: A structural and magnetic study. Inorg. Chem. 1998, 37, 2443–2449. [Google Scholar] [CrossRef]
- Li, Y.-T.; Yan, C.-W.; Wang, H.D. Synthesis, characterization, and magnetic study of μ-oxamido-bridged copper(II)–lanthanide(III) heterobinuclear complexes. Polish J. Chem. 2000, 74, 1655–1663. [Google Scholar]
- Li, Y.-T.; Yan, C.-W.; Zhang, J. Synthesis, characterization, and magnetic properties of copper(II)-lanthanide(III) heterobinuclear complexes bridged by N,N-oxamidobis(3,5-dibromobenzoato)cuprate(II). Crystal Growth Design 2003, 3, 481–485. [Google Scholar] [CrossRef]
- Shiga, T.; Ohba, M.; Okawa, H. Structure and magnetism of a trinuclear CuII–GdIII–CuII complex derived from one-pot reaction with 2,6-di(acetoacetyl)pyridine. Inorg. Chem. Commun. 2003, 6, 15–18. [Google Scholar] [CrossRef]
- Kido, T.; Ikuta, Y.; Sunatsuki, Y.; Ogawa, Y.; Matsumoto, N.; Re, N. Nature of copper(II)–lanthanide(III) magnetic interactions and generation of a large magnetic moment with magnetic anisotropy of 3d–4f cyclic cylindrical tetranuclear complexes [CuIILLnIII(hfac)2]2, (H3L = 1-(2-Hydroxybenzamido)-2-(2-hydroxy-3-methoxybenzylideneamino)ethane and Hhfac = hexafluoroacetylacetone, LnIII = Eu, Gd, Tb, Dy). Inorg. Chem. 2003, 42, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Sorace, L.; Casellato, U.; Tomasin, P.; Vigato, P.A. d- or f-Mononuclear and related heterodinuclear complexes with [1+1] asymmetric compartmental macrocycles. Eur. J. Inorg. Chem. 2004, 2004, 3887–3900. [Google Scholar] [CrossRef]
- Koner, R.; Lee, G.-H.; Wang, Y.; Wei, H.-H.; Mohanta, S. Two new diphenoxo-bridged discrete dinuclear CuIIGdIII compounds with cyclic diimino moieties: Synthesis, structure, and magnetic properties. Eur. J. Inorg. Chem. 2005, 2005, 1500–1505. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.-Y.; Zhao, B.; Yu, A.; Song, H.-B.; Cheng, P.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P. Construction of 3d-4f mixed-metal complexes based on a binuclear oxovanadium unit: synthesis, crystal structure, EPR, and magnetic properties. Inorg. Chem. 2006, 45, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Merca, A.; Müller, A.; Slageren, J.V.; Läge, M.; Krebs, B. Systematic study of the interaction between VIV centres and lanthanide ions MIII in well defined {VIV2MIII}{AsIIIW9O33}2 Sandwich Type Clusters: Part 1. J. Clust. Sci. 2007, 18, 711–719. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals. Chem. Rev. 2002, 102, 2369–2387, and reverences therein. [Google Scholar] [CrossRef] [PubMed]
- Tanase, S.; Reedijk, J. Chemistry and magnetism of cyanido-bridged d-f assemblies. Coord. Chem. Rev. 2006, 250, 2501–2510, and references therein. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A tetranuclear 3d-4f single molecular magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Trombé, J.C.; Gleizes, A.; Galy, J. Synthese et etude cristallochimique de dithio-oxalates mixtes hydrates de nickel et d elements de terres rares (t.r. (III) = Y, La, Ce, Nd, Sm, Eu, Gd, Dy, Er, Yb). Structure cristalline de Ni3Eu2(O2C2S2)6(H2O)10 xH2O (10<x<12). R. Acad. Sc. Paris 1982, 294-II, 1369–1372. [Google Scholar]
- Trombé, J.C.; Gleizes, A.; Galy, J. Structural evolution within a series of hydrated bis(dithiooxalato)nickelate(II) of rare earth (RE)2(H2O)2nNi3(S2C2O2)6, xH2O. Inorg. Chim. Acta 1984, 87, 129–141. [Google Scholar] [CrossRef]
- Siebold, M.; Kelling, A.; Schilde, U.; Strauch, P. Heterobimetallische 3d-4f Komplexe mit Bis(1,2-dithiooxalato)nickelat(II) als planarem Brückenbaustein. Z. Naturforsch. 2005, 60b, 1149–1157. [Google Scholar]
- Siebold, M.; Eidner, S.; Kelling, A.; Kumke, M.U.; Schilde, U.; Strauch, P. Pentanuclear heterobimetallic 3d-4f complexes – structure and luminescence. Z. anorg. allg. Chem. 2006, 632, 1963–1965. [Google Scholar] [CrossRef]
- Siebold, M.; Korabik, M.; Schilde, U.; Mrozinski, J.; Strauch, P. Pentanuclear heterobimetallic 3d-4f complexes of Ln2M3-type – structure and magnetism. Chem. Papers 2008, 62, 487–495. [Google Scholar] [CrossRef]
- Siebold, M.; Strauch, P. Series of Pentanuclear Heterobimetallic 3d-4f-Complexes Revisited. In Advances in Coordination, Bioinorganic and Inorganic Chemistry; Melnik, M., Šima, J., Tatarko, M., Eds.; Slovak Technical University Press: Bratislava, Slovakia, 2005; pp. 399–410. [Google Scholar]
- Czernuszewicz, R.S.; Nakamoto, K.; Strommen, D.P. Resonance raman spectra and vibrational assignments of “red” and “black” forms of potassium bis(dithiooxalato) nickel(II). J. Am. Chem. Soc. 1982, 104, 1515–1521. [Google Scholar] [CrossRef]
- X-Area; vers. 1.26; Stoe & Cie GmbH: Darmstadt, Germany, 2004.
- Sheldrick, G.M. SHELXS-97; Program for Crystal Structure Solution; University Göttingen: Göttingen Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014/7; Program for the Refinement of Crystal Structures; University Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Matz, C.; Mattes, R. Darstellung und Struktur von Kalium-1,2-diselenooxalat. Z. Naturforsch. 1978, 88b, 461–462. [Google Scholar]
- Wenzel, B.; Wehse, B.; Schilde, U.; Strauch, P. 1,2-Dithioquadratato- und 1,2-Dithiooxalatoindate(III). Z. anorg. allg. Chem. 2004, 630, 1469–1476. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).