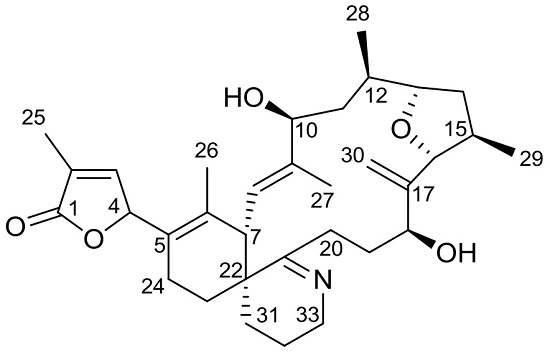
(5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo[2,3]cyclohexadeca[1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B)
Abstract
:1. Introduction
2. Results
3. Experimental Section
4. Discussion
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L.C. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Borkman, D.G.; Smayda, T.G.; Tomas, C.R.; York, R.; Strangman, W.K.; Wright, J.L.C. Toxic Alexandrium peruvianum (Balech and de Mendiola) Balech and Tangen in Narragansett Bay, Rhode Island (US). Harmful Algae 2012, 19, 92–100. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Massert, E.; Montressor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.; Riobó, P.; Rodríguez, F.; Franco, J.M.; Bravo, I. Differences in the toxin profiles of Alexandrium ostenfeldii (Dinophyceae) strains isolated from different geographic origins: Evidence of paralytic toxin, spirolide, and gymnodimine. Toxicon 2015, 103, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgó, J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Anttila, M.; Strangman, W.; York, R.; Tomas, C.; Wright, J.L.C. Biosynthetic studies of 13-desmethyl spirolide C produced by Alexandrium ostenfeldii (= A. peruvianum): Rationalization of the biosynthetic pathway following incorporation of 13C-labeled methionine and application of the odd-even rule of methylation. J. Nat. Prod. 2015. [Google Scholar] [CrossRef]
- Stivala, C.E.; Banoit, E.; Aráoz, R.; Servent, D.; Novikov, A.; Molgó, J.; Zakarian, A. Synthesis and biology of cyclic imine toxins, an emerging class of potent, globally distributed marine toxins. Nat. Prod. Rep. 2015, 32, 411–435. [Google Scholar] [CrossRef] [PubMed]
- Sleno, L.; Chalmers, M.J.; Volmer, D.A. Structural study of spirolide marine toxins by mass spectrometry. Part II. Mass spectrometric characterization of unknown spirolides and related compounds in a cultured phytoplankton extract. Anal. Bioanal. Chem. 2004, 378, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Sleno, L.; Windust, A.J.; Volmer, D.A. Structural study of spirolide marine toxins by mass spectrometry. Part I. Fragmentation pathways of 13-desmethyl spirolide C by collision-induced dissociation and infared multiphoton dissociation mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Harju, K.; Koskela, H.; Kremp, A.; Suikkanen, S.; de la Iglesia, P.; Miles, C.O.; Krock, B.; Vanninen, P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon 2016, 112, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. New analogue of gymnodimine from a Gymnodinium species. J. Agric. Food Chem. 2000, 48, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B from Karenia seliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Stewart, M.; Blunt, J.W.; Munro, M.H.G.; Robinson, W.T.; Hannah, D.J. The absolute stereochemistry of the New Zealand shellfish toxin gymnodimine. Tetrahedron Lett. 1997, 38, 4889–4890. [Google Scholar] [CrossRef]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. The cholinergic antagonist gymnodimine improves Aβ and tau neuropathology in an in vitro model of Alzheimer disease. Cell. Physiol. Biochem. 2011, 27, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M.; López, A.; González, C.V. Use of Gymnodimine Analogues and Derivatives for the Treatment and/or Prevention of Neurodegenerative Diseases Associated with Tau and β-Amyloid. U.S. Patent 20120245223 A1, 2 September 2010. [Google Scholar]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Aβ and hyperphosphorylated tau in vitro. Neurochem. Int. 2011, 59, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Ortero, P.; Vale, C.; Alphonso, A.; Antelo, A.; Giménez-Llort, L.; Chabaud, L.; Guillou, C.; Botana, L.M. Benefit of 13-desmethyl spirolide C treatment in triple transgenic mouse model of Alzheimer disease: Beta-amyloid and neuronal markers improvement. Curr. Alzheimer Res. 2013, 10, 279–289. [Google Scholar] [CrossRef] [PubMed]

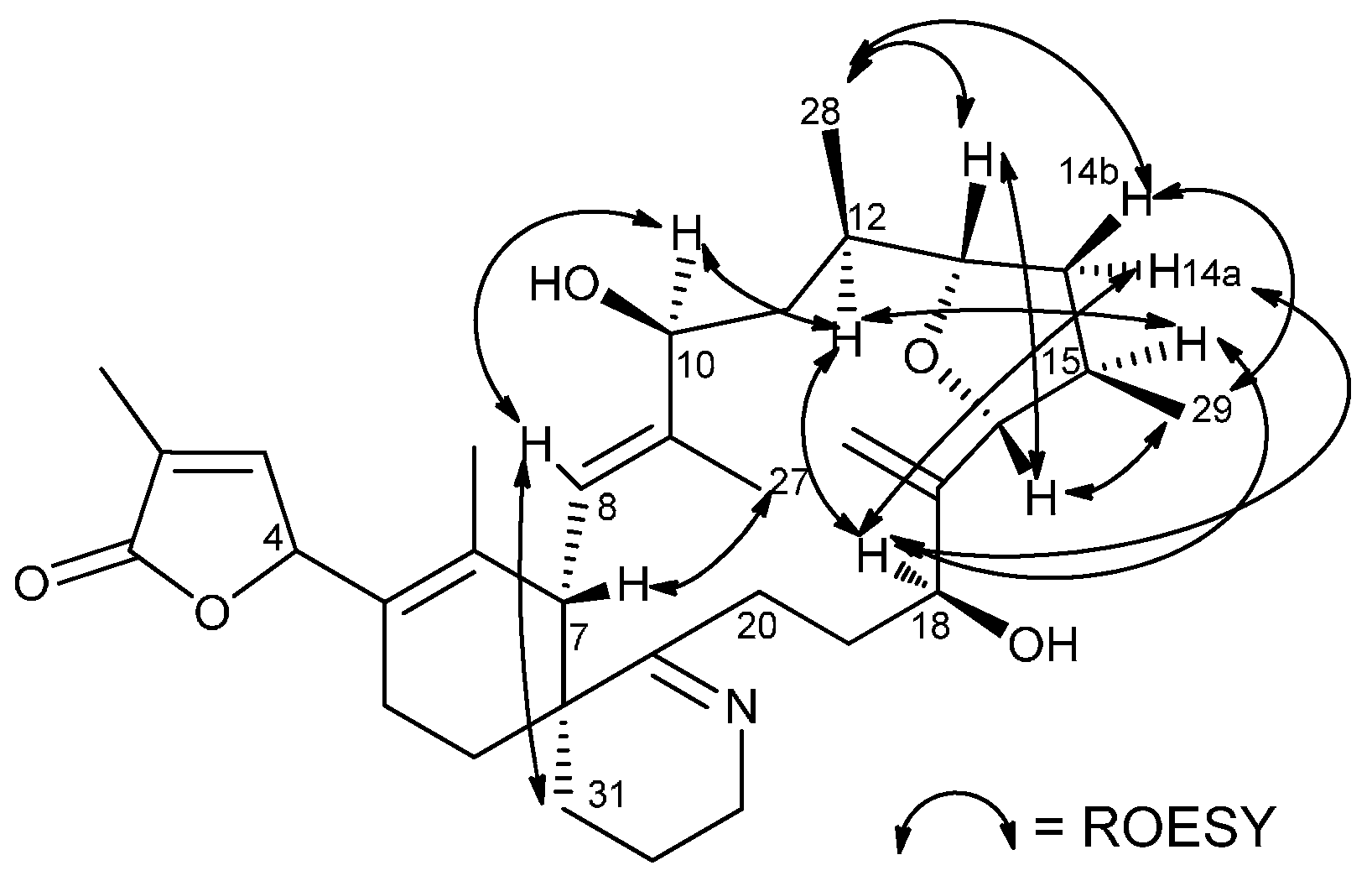
| Position | δH (mult, J in Hz) | δC (mult) | COSY | HMBC | ROESY |
|---|---|---|---|---|---|
| 1 | 175.3 C(q) | ||||
| 2 | 130.2 C(q) | ||||
| 3 | 7.15 (br s) | 148.8 CH | 4,25 | 1,2,4,25 | 25 |
| 4 | 5.95 (br s) | 81.3 CH | 3,25 | 2, 3,5, 6, 24 | 26 |
| 5 | 125.0 C(q) | ||||
| 6 | 134.1 C(q) | ||||
| 7 | 3.66 (m) | 46.1 CH | 8 | 6,8,9,22 | 20a,26,27 |
| 8 | 5.34 (d, 11.0) | 125.3CH | 7 | 7,10, 27,22 | 10, 26, 27, 31a,32a |
| 9 | 142.9 C(q) | ||||
| 10 | 4.52 (d, 10.5) | 77.2 CH | 11a,b | 8,11, 27 | 8, 11b, 12, 28 |
| 11a | 2.81 (t, 12.0) | 39.9 CH2 | 10, 11b, 12 | 9, 10,12, 13, 26 | 27 |
| 11b | 1.47 (m) | 10, 11a, 12 | 9, 10,12, 26 | 10, 13 | |
| 12 | 1.40 (m) | 35.7 CH | 11a,b, 28 | 11,13, 28 | 10, 15, 18 |
| 13 | 3.71 (m) | 83.6 CH | 12, 14a,b | 11,15,16, 28 | 11b,14b, 16, 28 |
| 14a | 2.15 (m) | 13,15 | 15,16, 29 | 15,18 | |
| 14b | 1.58 (m) | 38.4 CH2 | 13,15 | 15,16, 29 | 13, 16, 28, 29 |
| 15 | 2.94 (m) | 36.8 CH | 14 a,b, 16, 29 | 14,16,17, 29 | 12, 14a,18,19a |
| 16 | 4.08 (d, 8.5) | 92.1 CH | 15 | 15,17, 18, 29, 30 | 13, 14b, 29 |
| 17 | 156.3 C(q) | ||||
| 18 | 4.35 (d, 10.0) | 70.9 CH | 19a,b | 16, 17, 19,20,30 | 12,14a,15,19a |
| 19a | 2.58 (m) | 18, 19b, 20a,b | 18, 20,21 | 15, 18 | |
| 19b | 2.04 (m) | 34.6 CH2 | 18, 19a, 20a,b | 18, 20,21 | |
| 20a | 2.98 (m) | 31.1 CH2 | 19a,b, 20b | 18, 19,21 | 7 |
| 20b | 2.37 (m) | 19a,b, 20a | 18, 19,21 | ||
| 21 | 174.3 C(q) | ||||
| 22 | 41.9 C(q) | ||||
| 23a | 1.71 (m) | 32.9 CH2 | 23b, 24 a,b | 5,7,24,31 | |
| 23b | 1.27 (m) | 23a | 5,7,21,22,24,31 | ||
| 24a | 2.04 (m) | 19.8 CH2 | 23a, 24b | ||
| 24b | 1.59 (m) | 23a, 24a | 5,6,22 | ||
| 25 | 2.00 (s) | 11.0 CH3 | 3,4 | 1, 2, 3, 4 | 3 |
| 26 | 1.66 (s) | 17.0 CH3 | 6, 7, 8 | 4,7 | |
| 27 | 2.14 (s) | 12.0 CH3 | 7, 8, 9, 10 | 7,11a | |
| 28 | 0.96 (d; 6.5) | 17.3 CH3 | 12 | 11,12,13 | 10,13,14b |
| 29 | 1.06 (d; 6.5) | 17.6 CH3 | 15 | 14,15,16 | 14b, 16 |
| 30a | 5.55 (m) | 112.5 CH2 | 30b | 16,17,18 | |
| 30b | 5.11 (m) | 30a | 16,17,18 | ||
| 31a | 1.83 (m) | 26.4 CH2 | 31b, 32a,b | 7, 21, 22,23 | 8 |
| 31b | 1.35 (m) | 31a, 32a,b | 7, 21, 22,23 | ||
| 32a | 1.58 (m) | 21.3 CH2 | 31a, 32b, 33a,b | 22,33 | |
| 32b | 1.44 (m) | 31a, 32a, 33a,b | 22,33 | ||
| 33a | 3.64 (m) | 49.5 CH2 | 32a,b, 33b | 21, 31 | |
| 33b | 3.45 (m) | 32a,b, 33a | 21, 31 |
| Position | 12-Methyl Gym B δH | 12-Methyl Gym B δC | 12-Methyl Gym δH | 12-Methyl Gym δC |
|---|---|---|---|---|
| 1 | 175.3 | 175.4 | ||
| 2 | 130.2 | 130.3 | ||
| 3 | 7.15 | 148.8 | 7.13 | 148.7 |
| 4 | 5.95 | 81.3 | 5.95 | 81.4 |
| 5 | 125.0 | 125.3 | ||
| 6 | 134.1 | 134.1 | ||
| 7 | 3.66 | 46.1 | 3.63 | 46.6 |
| 8 | 5.34 | 125.3 | 5.34 | 126.2 |
| 9 | 142.9 | 141.7 | ||
| 10 | 4.52 | 77.2 | 4.58 | 77.3 |
| 11a | 2.81 | 39.9 | 2.92 | 40.3 |
| 11b | 1.47 | 1.61 | ||
| 12 | 1.40 | 35.7 | 1.52 | 37.1 |
| 13 | 3.71 | 83.6 | 3.84 | 83.7 |
| 14a | 2.15 | 38.4 | 1.78 | 36.0 |
| 14b | 1.58 | 1.52 | ||
| 15 | 2.94 | 36.8 | 2.14 | 38.6 |
| 16 | 4.08 | 92.1 | 4.12 | 89.8 |
| 17 | 156.3 | 134.5 | ||
| 18 | 4.35 | 70.9 | 5.40 | 126.8 |
| 19a | 2.58 | 34.6 | 2.77 | 22.8 |
| 19b | 2.04 | 2.11 | ||
| 20a | 2.98 | 31.1 | 2.65 | 31.4 |
| 20b | 2.37 | 2.48 | ||
| 21 | 174.3 | 171.8 | ||
| 22 | 41.9 | 41.8 | ||
| 23a | 1.71 | 32.9 | 1.71 | 34.4 |
| 23b | 1.27 | 1.32 | ||
| 24a | 2.04 | 19.8 | 2.06 | 20.0 |
| 24b | 1.59 | 1.59 | ||
| 25 | 2.00 | 11.0 | 2.00 | 11.1 |
| 26 | 1.66 | 17.0 | 1.69 | 17.0 |
| 27 | 2.14 | 12.0 | 2.10 | 11.7 |
| 28 | 0.96 | 17.3 | 0.92 | 17.0 |
| 29 | 1.06 | 17.6 | 1.06 | 20.6 |
| 30a | 5.55 | 112.5 | 1.54 | 14.9 |
| 30b | 5.11 | |||
| 31a | 1.83 | 26.4 | 1.89 | 27.4 |
| 31b | 1.35 | 1.32 | ||
| 32a | 1.58 | 21.3 | 1.54 | 21.4 |
| 32b | 1.44 | 1.47 | ||
| 33a | 3.64 | 49.5 | 3.75 | 50.6 |
| 33b | 3.45 | 3.40 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strangman, W.; Anttila, M.; Tomas, C.; Wright, J.L.C. (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo[2,3]cyclohexadeca[1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B). Molbank 2016, 2016, M896. https://doi.org/10.3390/M896
Strangman W, Anttila M, Tomas C, Wright JLC. (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo[2,3]cyclohexadeca[1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B). Molbank. 2016; 2016(2):M896. https://doi.org/10.3390/M896
Chicago/Turabian StyleStrangman, Wendy, Matthew Anttila, Carmelo Tomas, and Jeffrey L. C. Wright. 2016. "(5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo[2,3]cyclohexadeca[1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B)" Molbank 2016, no. 2: M896. https://doi.org/10.3390/M896
APA StyleStrangman, W., Anttila, M., Tomas, C., & Wright, J. L. C. (2016). (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-tetramethyl-18-methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-hexadecahydro-2H-14,17-epoxybenzo[2,3]cyclohexadeca[1,2-b]pyridine-7-yl]-3-methylfuran-2(5H)-one (12-Methylgymnodimine B). Molbank, 2016(2), M896. https://doi.org/10.3390/M896