Abstract
A novel hybrid consisting of quinazoline and adenine moieties has been synthesized as a precursor of a potential biologically active target compound. The structure of 9-(4-methoxyquinazolin-2-yl)-9H-purin-6-amine (2) was characterized and confirmed using the following spectroscopic methods: LC-UV-MS, 1H-NMR, 13C-NMR and HSQC-NMR.
1. Introduction
Various N-aryl nucleobases are known for their antitumor [1,2] and antimicrobial [3] activity. A significant number of N9-arylpurines have been reported to act as agonists or antagonists for several receptors and enzymes [4]. In particular, a variety of 9-substituted adenines have been published for their interaction with adenosine receptors, which are found to be upregulated in various tumor cells [5]. On the other hand, quinazolines as heterocyclic compounds containing two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring [6], are considered to be a "privileged structure" for drug development [7]. Over the years, medicinal chemists have synthesized a variety of quinazoline derivatives with different biological activities (anti-cancer [8,9,10,11], antimicrobial [12,13,14,15], etc.) by inserting various active groups to the quinazoline moiety using developing synthetic methods [16].
Taking into consideration the value of both adenine and quinazoline entities, we have designed a route for the synthesis of a target compound consisting the conjugation of these moieties. In silico modeling of the coupled molecule which assess the binding affinity as well as the potential inhibitory effect on specific binding sites of human proteins, reveals a promising result that this compound can be used as precursor molecule for medicinal chemical structures. This coupling is achieved by regioselective N-arylation via nucleophilic aromatic substitution (SNAr) [17]. In this paper we report the synthesis of 9-(4-methoxyquinazolin-2-yl)-9H-purin-6-amine which constitutes an intermediate of the aforementioned route (Scheme 1).
2. Experimental Section
2.1. General Methods
All reagents were used as received without further purification unless stated otherwise. Flash chromatography was performed on silica gel 60, 0.04–0.063 mm (Zeochem, Uetikon, Switzerland). Melting points were measured on a Stuart SMP30 melting point apparatus (Bibby Scientific Limited (Group HQ), Stone, UK) and are uncorrected. HPLC was carried out on an Agilent 1200 system (Agilent Technology, Santa Clara, CA, USA), using the column: Grace Prevail 5 μm C18, 4.6 mm × 250 mm (Grace Alltech, Breda, NL, USA). Mass analysis was performed on a Bruker MicrOTOF (Bruker Daltonics, Bremen, Germany). ESI was used for ionization and the spectrum was recorded in positive mode. NMR spectra (1H, 13C, 13C-HSQC) were recorded on a Bruker 500 MHz Avance III system (Bruker Biospin, Karlsruhe, Germany) equipped with 5 mm cryoprobe, CPTCI (1H-13C/15N/2H + Z-gradients). Chemical shifts are given in ppm and J values in Hertz (Hz). Solids were dried in a Memmert UNB 400 oven (Memmert GmbH, Schwabach, Germany).
2-chloro-4-methoxyquinazoline (1) has been synthesized according to reported methods [18,19]. Spectroscopic results and physical properties are consistent with the literature [20].
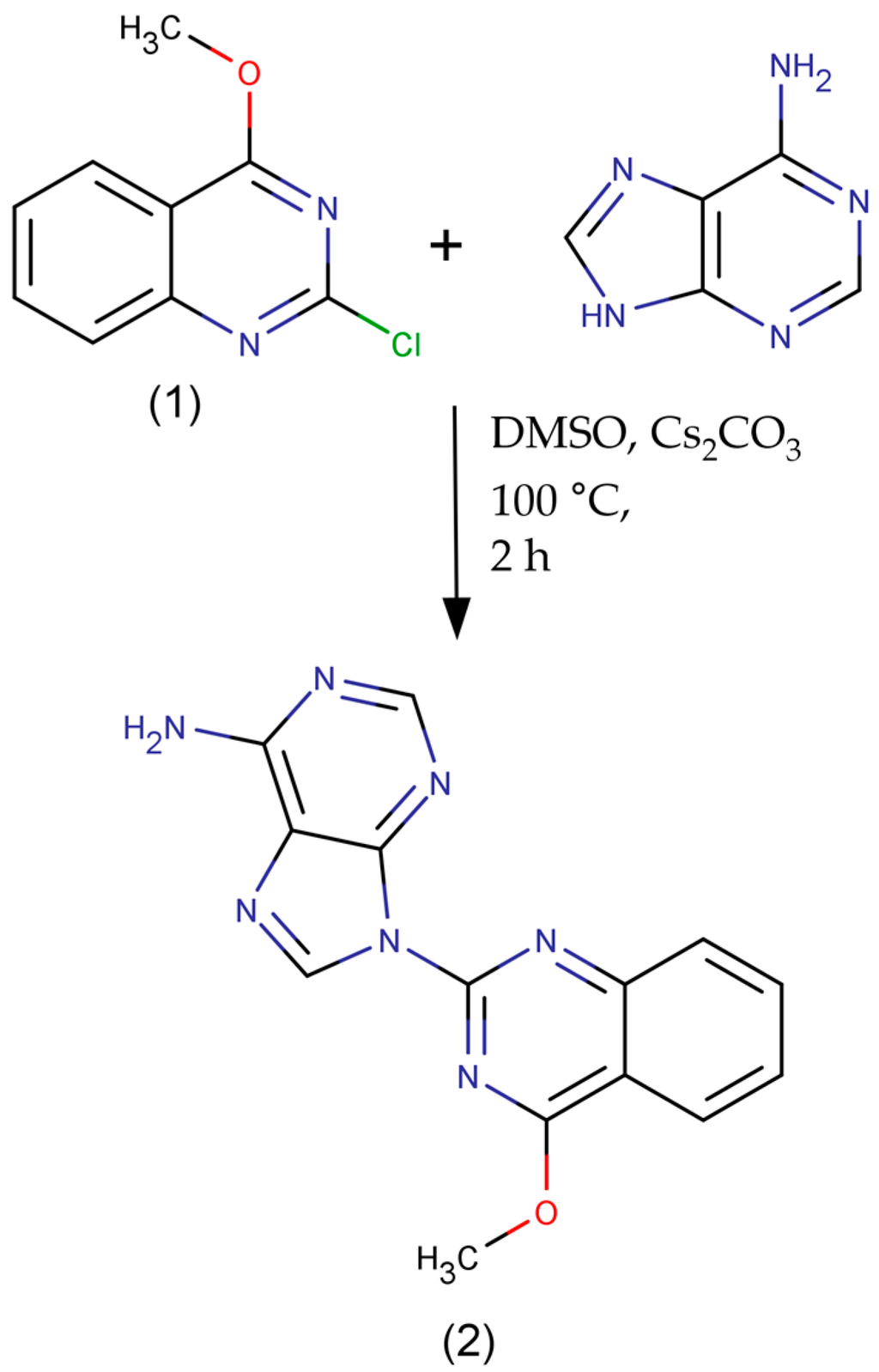
Scheme 1.
The synthesis of 9-(4-methoxyquinazolin-2-yl)-9H-purin-6-amine.
2.2. Synthesis of 9-(4-Methoxyquinazolin-2-yl)-9H-purin-6-amine (2)
To a well-grounded mixture of adenine (1.3512 g, 10 mmol), 2-chloro-4-methoxyquinazoline (2.5104 g, 13 mmol), cesium carbonate (3.2612 g, 10 mmol) and silica gel (3.5 g) was added 20 mL of dry DMSO under inert atmosphere. The solution was stirred at 100 °C for 2 h and then it was poured into ice cold water and filtered. The desired product was anchored on the silica gel and was purified by flash chromatography. On elution with chloroform-methanol 7:1 (Rf = 0.38), 9-(4-methoxyquinazolin-2-yl)-9H-purin-6-amine was obtained as light yellow solid (m.p. = 303 °C) in 37% yield. The relatively modest yield was expected as 2-chloro-4-methoxyquinazoline is considered a non-activated substrate for nucleophilic aromatic substitution.
1H-NMR (500 MHz, CDCl3) δ 4.36 (s, J = 2.99 Hz, 3H, OCH3), 7.65 (t, J = 1.00 Hz, 1H, Ar-H), 7.87 (d, J = 0.99 Hz, 1H, Ar-H), 7.96 (t, J = 1.00 Hz, 1H, Ar-H), 8.25 (d, J = 0.99 Hz, 1H, Ar-H), 8.41 (s, J = 0.98 Hz, 1H, N=CH-N), 9.38 (s, J = 0.98 Hz, 1H, N=CH-N); 13C-NMR (500 MHz, CDCl3) δ 55.6 (C=O), 110.0 (C12), 115.1 (C3), 124.6 (C7), 126.1 (C5), 127.7 (C4), 135.7 (C6), 145.4 (C14), 150.1 (C8), 150.4 (C11), 152.9 (C13), 154.3 (C10), 161.3 (C2), 169.2 (C9); LC-MS (ESI): [M + H]+ calculated for C14H11N7O = 294.1094; found 294.1090. Copies of all spectra (Figures S1–S7) are provided in the Supplementary Materials.
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4The molefiles and the other supplementary materials can be found at http://www.mdpi.com/1422-8599/2016/1/M885.
Author Contributions
All authors contributed equally to both experimental and writing work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koppel, H.C.; Robins, R.K. Potential Purine Antagonists. XI. Synthesis of some 9-aryl(alkyl)-2,6-disubstituted purines. J. Am. Chem. Soc. 1958, 80, 2751–2755. [Google Scholar] [CrossRef]
- Koppel, H.C.; O’Brien, D.E.; Robins, R.K. Potential Purine Antagonists. XIX. Synthesis of Some 9-Alkyl (aryl)-2-amino-6-substituted Purines and Related V-Triazolo [d] pyrimidines. J. Am. Chem. Soc. 1959, 81, 3046–3051. [Google Scholar] [CrossRef]
- Basyouni, W.; Hosni, H.; Helmy, S. Synthesis and antimicrobial activity of some new 6-substituted 9-arylpurine derivatives. Egypt. J. Chem. 1999, 42, 587–598. [Google Scholar]
- Thompson, R.D.; Secunda, S.; Daly, J.W.; Olsson, R.A. N6,9-Disubstituted adenines: Potent, selective antagonists at the A1 adenosine receptor. J. Med. Chem. 1991, 34, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.; Bar-Yehuda, S.; Synowitz, M.; Powell, J.D.; Klotz, K.N.; Gessi, S.; Borea, P.A. Adenosine Receptors and Cancer. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Springer: Berlin, Germany, 2009; Volume 193, pp. 399–441. [Google Scholar]
- Armarego, W.L.F.; Hitchings, G.H.; Elio, G.B. Quinazolines. In The Chemistry of Heterocyclic Compounds, Fused Pyrimidines., Part. I; Wiley and Sons: Chichester, UK, 1967; Volume 24, pp. 11–38. [Google Scholar]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Chandregowda, V.; Kush, A.K.; Chandrasekara Reddy, G. Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur. J. Med. Chem. 2009, 44, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashood, S.T.; Aboldahab, I.A.; Nagi, M.N.; Abouzeid, L.A.; Abdel-Aziz, A.A.; Abdel-Hamide, S.G.; Youssef, K.M.; Al-Obaid, A.M.; El-Subbagh, H.I. Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg. Med. Chem. 2006, 14, 8608–8621. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Dorff, P.N.; Gibbs, A.R.; Nandanan, E.; Reid, L.M.; Neil, J.P.; VanBrocklin, H.F. Synthesis of 6-acrylamido-4-(2-[18F]fluoroanilino)quinazoline: A prospective irreversible EGFR binding probe. J. Lablelled Compd. Rad. 2005, 48, 109–115. [Google Scholar] [CrossRef]
- Wakeling, A.E.; Guy, S.P.; Woodburn, J.R.; Ashton, S.E.; Curry, B.J.; Barker, A.J.; Gibson, K.H. ZD1839 (Iressa): An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002, 62, 5749–5754. [Google Scholar] [PubMed]
- Rohini, R.; Muralidhar Reddy, P.; Shanker, K.; Hu, A.; Ravinder, V. Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c]quinazolines. Eur. J. Med. Chem. 2010, 45, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Antipenko, L.; Karpenko, A.; Kovalenko, S.; Katsev, A.; Komarovska-Porokhnyavets, E.; Novikov, V.; Chekotilo, A. Synthesis of new 2-thio-[1,2,4]triazolo[1,5-c]quinazoline derivatives and its antimicrobial activity. Chem. Pharm. Bull. 2009, 57, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Jatav, V.; Kashaw, S.; Mishra, P. Synthesis and antimicrobial activity of some new 3–[5-(4-substituted)phenyl-1,3,4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-ones. Med. Chem. Res. 2008, 17, 205–211. [Google Scholar] [CrossRef]
- Aly, A.A. Synthesis of novel quinazoline derivatives as antimicrobial agents. Chin. J. Chem. 2003, 21, 339–346. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Khalafi-Nezland, A.; Zare, A.; Parhami, A. Regioselective N-arylation of some pyrimidine and purine nucleobases. Syn. Commun. 2006, 36, 3549–3562. [Google Scholar] [CrossRef]
- Lange, N.A.; Sheibley, F.E. Quinazolines. II. The interaction of 2,4-dichloroquinazoline in alcohol with salts and bases. J. Am. Chem. Soc. 1931, 53, 3867–3875. [Google Scholar] [CrossRef]
- Lange, N.A.; Roush, W.E.; Asbeck, H.J. Quinazolines. I. The interaction of 2,4-dichloroquinazoline with sodium alcoholates and sodium phenates with the replacement of one halogen to form halogen-oxygen ethers. J. Am. Chem. Soc. 1930, 52, 3696–3702. [Google Scholar] [CrossRef]
- Almirall, S.A.; Eastwood, P.R.; Gonzalez Rodriguez, J.; Bach Tana, J.; Pages Santacana, L.M.; Taltavull Moll, J.; Caturla Javaloyes, J.F.; Matassa, V.G. Imidazopyridine Derivatives as JAK Inhibitors. Patent WO2011/76419 A1, 29 June 2011. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).