4,5-Dimethylbenzene-1,2-dimethanol
Abstract
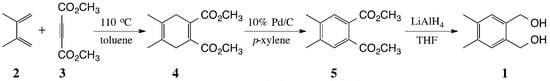
:Experimental
4,5-Dimethylbenzene-1,2-dimethanol (1)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Bourne, C.R.; Bunce, R.A.; Bourne, P.C.; Berlin, K.D.; Barrow, E.W.; Barrow, W.W. Crystal structure of Bacillus anthracis dihydrofolate reductase with the dihydrophthalazine-based trimethoprim derivative RAB1 provides a structural explanation of potency and selectivity. Antimicrob. Agents Chemother. 2009, 53, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Bourne, C.R.; Barrow, E.W.; Bunce, R.A.; Bourne, P.C.; Berlin, K.D.; Barrow, W.W. Inhibition of antibiotic-resistant Staphylococcus aureus by the broad-spectrum dihydrofolate reductase inhibitor RAB1. Antimicrob. Agents Chemother. 2010, 54, 3825–3833. [Google Scholar] [CrossRef] [PubMed]
- Nammalwar, B.; Bunce, R.A.; Berlin, K.D.; Bourne, C.R.; Bourne, P.C.; Barrow, E.W.; Barrow, W.W. Synthesis and biological activity of substituted 2,4-diaminopyrimidines that inhibit Bacillus anthracis. Eur. J. Med. Chem. 2012, 54, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Nammalwar, B.; Bourne, C.R.; Bunce, R.A.; Wakeham, N.; Bourne, P.C.; Ramnarayan, K.; Mylvaganam, S.; Berlin, K.D.; Barrow, E.W.; Barrow, W.W. Inhibition of bacterial dihydrofolate reductase by 6-alkyl-2,4-diaminopyrimidines. ChemMedChem 2012, 7, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Bourne, C.R.; Wakeham, N.; Nammalwar, B.; Tseitin, V.; Bourne, P.C.; Barrow, E.W.; Mylvaganam, S.; Ramnarayan, K.; Bunce, R.A.; Berlin, K.D.; Barrow, W.W. Structure-activity relationship for enantiomers of potent inhibitors of B. anthracis dihydrofolate reductase. Biochim. Biophys. Acta Proteins Proteomics 2013, 1834, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Bourne, C.R.; Wakeham, N.; Webb, N.; Nammalwar, B.; Bunce, R.A.; Berlin, K.D.; Barrow, W.W. The structure and competitive substrate inhibition of dihydrofolate reductase from Enterococcus faecalis reveal restrictions to cofactor docking. Biochemistry 2014, 53, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Brickwood, D.J.; Ollis, W.D.; Stephanatou, J.S.; Stoddart, J.F. Conformational behaviour of medium-sized rings. Part 6. 5,6,11,12,17,18-Hexahydrotribenzo[a,e,i]cyclododecene and its 2,3,8,9,14,15- and 1,4,7,10,13,16-hexamethyl derivatives. 2,3,8,9- and 1,4,7,10-Tetramethyl-5,6,11,12-tetrahydrodibenzo[a,e]cyclo-octene. J. Chem. Soc. Perkin Trans. 1 1978, 1398–1414. [Google Scholar] [CrossRef]
- Farooq, O. Oxidation of aromatic 1,2-dimethanols by activated dimethyl sulfoxide. Synthesis 1994, 1035–1036. [Google Scholar] [CrossRef]
- Fu, P.P.; Harvey, R.G. Dehydrogenation of polycyclic hydroaromatic compounds. Chem. Rev. 1978, 78, 317–361. [Google Scholar] [CrossRef]
- Kanamitsu, N.; Osaki, T.; Itsuji, Y.; Yoshimura, M.; Tsujimoto, H.; Soga, M. Novel water-soluble sedative-hypnotic agents: isoindolin-1-one derivatives. Chem. Pharm. Bull. 2007, 55, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Hennige, H.; Kreher, R.P.; Konrad, M.; Jelitto, F. Studies on the chemistry of isoindoles and isoindolenines. XXVII. 3-Alkoxy-1H-isoindoles: Syntheses and properties. Chem. Ber. 1988, 121, 243–252. [Google Scholar] [CrossRef]
- Stapleton, G.; White, A.I. Synthesis of methyl-substituted acridinecarboxylic acids. J. Am. Pharm. Assoc. (1912–1977) 1954, 43, 193–200. [Google Scholar] [CrossRef]
- Jagtap, S.P.; Mukhopadhyay, S.; Coropceanu, V.; Brizius, G.L.; Bredas, J.-L.; Collard, D.M. Closely stacked oligo(phenylene ethynylene)s: Effect of π-stacking on the electronic properties of conjugated chromophores. J. Am. Chem. Soc. 2012, 134, 7176–7185. [Google Scholar] [CrossRef] [PubMed]
- IR: 1725 cm−1; 1H NMR (400 MHz, CDCl3): δ 3.78 (s, 6H), 2.92 (s, 4H), 1.66 (s, 6H); 13C NMR (100 MHz, CDCl3): δ 168.42, 132.76, 121.53, 52.16, 34.13, 17.96.
- The optimization results for the dehydrogenation of 4 to 5 on a 1-g scale are summarized in the table below. Since the 4 and 5 are chromatographically difficult to separate, it is necessary to get complete conversion to 5. This occurs most efficiently and economically using 5 wt % of 10% Pd-C in refluxing p-xylene for 24 h.
Table 1. Optimization for the dehydrogenation of 4 to 5. wt % of 10% Pd-C Time (h) Yield of 5 (%) 20 6 92 15 10 91 10 16 90 5 24 89 2.5 48 72 a a Some starting material 4 remained after 48 h. - IR: 1729 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.49 (s, 2H), 3.88 (s, 6H), 2.31 (s, 6H); 13C NMR (CDCl3, 100 MHz): δ 168.24, 140.22, 130.02, 129.39, 52.43, 19.62.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnanasekaran, K.K.; Bunce, R.A.; Berlin, K.D. 4,5-Dimethylbenzene-1,2-dimethanol. Molbank 2014, 2014, M835. https://doi.org/10.3390/M835
Gnanasekaran KK, Bunce RA, Berlin KD. 4,5-Dimethylbenzene-1,2-dimethanol. Molbank. 2014; 2014(4):M835. https://doi.org/10.3390/M835
Chicago/Turabian StyleGnanasekaran, Krishna Kumar, Richard A. Bunce, and K. Darrell Berlin. 2014. "4,5-Dimethylbenzene-1,2-dimethanol" Molbank 2014, no. 4: M835. https://doi.org/10.3390/M835
APA StyleGnanasekaran, K. K., Bunce, R. A., & Berlin, K. D. (2014). 4,5-Dimethylbenzene-1,2-dimethanol. Molbank, 2014(4), M835. https://doi.org/10.3390/M835