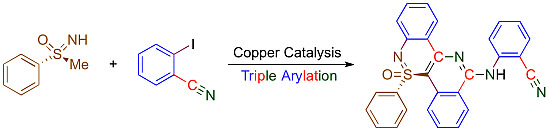
(R)-(−)-2-[(5-Oxido-5-phenyl-5λ4-isoquino[4,3-c][2,1]benzothiazin-12-yl)amino]benzonitrile
Abstract
:Introduction
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Bolm, C.; Simić, O. Highly Enantioselective Hetero-Diels-Alder Reactions Catalyzed by a C2-Symmetric Bis(sulfoximine) Copper(II) Complex. J. Am. Chem. Soc. 2001, 123, 3830–3831. [Google Scholar] [CrossRef] [PubMed]
- Langner, M.; Bolm, C. C1-Symmetric Sulfoximines as Ligands in Copper-Catalyzed Asymmetric Mukaiyama-Type Aldol Reactions. Angew. Chem. Int. Ed. 2004, 43, 5984–5987. [Google Scholar] [CrossRef] [PubMed]
- Moessner, C.; Bolm, C. Diphenylphosphanylsulfoximines as Ligands in Iridium-Catalyzed Asymmetric Imine Hydrogenations. Angew. Chem. Int. Ed. 2005, 44, 7564–7567. [Google Scholar] [CrossRef] [PubMed]
- Sedelmeier, J.; Hammerer, T.; Bolm, C. C1-Symmetric Oxazolinyl Sulfoximines as Ligands in Copper-Catalyzed Asymmetric Mukaiyama Aldol Reactions. Org. Lett. 2008, 10, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-M.; Bolm, C. Synthesis of Sulfoximine-Derived P,N Ligands and their Applications in Asymmetric Quinoline Hydrogenations. Adv. Synth. Catal. 2008, 350, 1101–1105. [Google Scholar] [CrossRef]
- Frings, M.; Atodiresei, I.; Wang, Y.; Runsink, J.; Raabe, G.; Bolm, C. C1-Symmetric Aminosulfoximines in Copper-Catalyzed Asymmetric Vinylogous Mukaiyama Aldol Reactions. Chem. Eur. J. 2010, 16, 4577–4587. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Hildebrand, J.P. Palladium-Catalyzed Carbon-Nitrogen Bond Formation: A Novel, Catalytic Approach towards N-Arylated Sulfoximines. Tetrahedron Lett. 1998, 39, 5731–5734. [Google Scholar] [CrossRef]
- Bolm, C.; Hildebrand, J.P. Palladium-Catalyzed N-Arylation of Sulfoximines with Aryl Bromides and Aryl Iodides. J. Org. Chem. 2000, 65, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Harmata, M.; Hong, X.; Ghosh, S.K. Microwave-Assisted N-Arylation of a Sulfoximine with Aryl Chlorides. Tetrahedron Lett. 2004, 45, 5233–5236. [Google Scholar] [CrossRef]
- Sedelmeier, J.; Bolm, C. Efficient Copper-Catalyzed N-Arylation of Sulfoximines with Aryl Iodides and Aryl Bromides. J. Org. Chem. 2005, 70, 6904–6906. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Kammel, G. Einfache und ergiebige Darstellungsmethoden für N-alkylierte Sulfoximide (Alkylimino-oxo-dialkyl-sulfurane). Chem. Ber. 1971, 104, 3234–3240. [Google Scholar] [CrossRef]
- CCDC 1014340 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- Sheldrick, G.M. SHELXS/L-97, Programs for the Solution and Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef] [PubMed]


© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Frings, M.; Atodiresei, I.; Bolm, C. (R)-(−)-2-[(5-Oxido-5-phenyl-5λ4-isoquino[4,3-c][2,1]benzothiazin-12-yl)amino]benzonitrile. Molbank 2014, 2014, M834. https://doi.org/10.3390/M834
Frings M, Atodiresei I, Bolm C. (R)-(−)-2-[(5-Oxido-5-phenyl-5λ4-isoquino[4,3-c][2,1]benzothiazin-12-yl)amino]benzonitrile. Molbank. 2014; 2014(3):M834. https://doi.org/10.3390/M834
Chicago/Turabian StyleFrings, Marcus, Iuliana Atodiresei, and Carsten Bolm. 2014. "(R)-(−)-2-[(5-Oxido-5-phenyl-5λ4-isoquino[4,3-c][2,1]benzothiazin-12-yl)amino]benzonitrile" Molbank 2014, no. 3: M834. https://doi.org/10.3390/M834
APA StyleFrings, M., Atodiresei, I., & Bolm, C. (2014). (R)-(−)-2-[(5-Oxido-5-phenyl-5λ4-isoquino[4,3-c][2,1]benzothiazin-12-yl)amino]benzonitrile. Molbank, 2014(3), M834. https://doi.org/10.3390/M834