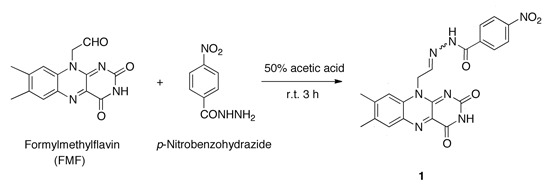
N'-[2-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethylidene]-4-nitrobenzohydrazide
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-L.; Kamata, H.; Karin, M. IKK/NF-κB signaling: balancing life and death-a new approach to cancer therapy. J. Clin. Invest. 2005, 115, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Sung, B.; Aggarwal, B.B. Nuclear factor-κB activation: from bench to bedside. Exp. Biol. Med. 2008, 233, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Visekruna, A.; Joeris, T.; Seidel, D.; Kroesen, A.; Loddenkemper, C.; Zeitz, M.; Kaufmann, S.H.E.; Schmidt-Ullrich, R.; Steinhoff, U. Proteasome-mediated degradation of IκBα and processing of p105 in Crohn disease and ulcerative colitis. J. Clin. Invest. 2006, 116, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yoshimori, A.; Kino, K.; Komori, R.; Miyazawa, H.; Tanuma, S.-I. A new small molecule that directly inhibits the DNA binding of NF-κB. Bioorg. Med. Chem. 2009, 17, 5293–5297. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kobayashi, T.; Nakatsu, A.; Miyazawa, H.; Kagechika, H. Synthesis and structure–activity relationship study of triazine-based inhibitors of the DNA binding of NF-κB. Chem. Pharm. Bull. 2014, 62, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Kino, K.; Kobayashi, T.; Arima, E.; Komori, R.; Kobayashi, T.; Miyazawa, H. Photoirradiation products of flavin derivatives, and the effects of photooxidation on guanine. Bioorg. Med. Chem. Lett. 2009, 19, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Kino, K.; Miyazawa, H.; Sugiyama, H. User-friendly synthesis and photoirradiation of a flavin-linked oligomer. Genes Environ. 2007, 29, 23–28. [Google Scholar] [CrossRef]


© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, M.; Kino, K.; Asada, E.; Katagiri, K.; Mori-Yasumoto, K.; Suzuki, M.; Kobayashi, T.; Miyazawa, H. N'-[2-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethylidene]-4-nitrobenzohydrazide. Molbank 2014, 2014, M836. https://doi.org/10.3390/M836
Morikawa M, Kino K, Asada E, Katagiri K, Mori-Yasumoto K, Suzuki M, Kobayashi T, Miyazawa H. N'-[2-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethylidene]-4-nitrobenzohydrazide. Molbank. 2014; 2014(4):M836. https://doi.org/10.3390/M836
Chicago/Turabian StyleMorikawa, Masayuki, Katsuhito Kino, Eriko Asada, Kosuke Katagiri, Kanami Mori-Yasumoto, Masayo Suzuki, Takanobu Kobayashi, and Hiroshi Miyazawa. 2014. "N'-[2-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethylidene]-4-nitrobenzohydrazide" Molbank 2014, no. 4: M836. https://doi.org/10.3390/M836
APA StyleMorikawa, M., Kino, K., Asada, E., Katagiri, K., Mori-Yasumoto, K., Suzuki, M., Kobayashi, T., & Miyazawa, H. (2014). N'-[2-(7,8-Dimethyl-2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl)ethylidene]-4-nitrobenzohydrazide. Molbank, 2014(4), M836. https://doi.org/10.3390/M836