Abstract
The title compound (3) is synthesized by the nucleophilic substitution of 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline 2 with 4-amino acetophenone in n-butanol. Newly prepared imiquimod derivative (3) is characterized by IR, NMR and mass spectral data.
Introduction
The quinoline scaffold is prevalent in a variety of pharmacologically active synthetic and natural compounds [1]. Imidazoquinoline is a double cyclic organic molecule; its derivatives and compounds are synthetic immunomodulatory drugs that act by binding toll-like receptors 7 and 8 (TLR7/TLR8) on dendritic cells [2,3]. Imidazoquinoline amine compounds are now known to be the first small low-molecular-weight immune response modifiers that function through the TLR receptors have potent anti-viral, anti-tumour, non-xanthine adenosine antagonist properties. The biological activity associated with imiquimod has been attributed to its induction of interferon (IFN)-alpha [4,5,6,7]. The various biological importance of imiquimod has prompted us to design and synthesize new structural analogue of imiquimod.
Results and Discussion
Several methods are known in the art of making 1H-imidazo[4,5-c]quinoline 4-amines and its derivatives, including Imiquimod. Literature revealed that amination of 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline can be carried out by three ways; The first is by nucleophilic substitution of a leaving group, the second is by reacting 1-isobutyl-1H-imidazo[4,5-c]quinoline-N-oxide with ammonium hydroxide or ammonium salts in presence of tosyl chloride at 0–5 °C and third is by reacting 1-isobutyl-1H-imidazo[4,5-c]quinoline-N-oxide with benzoyl chloride [8].
The title compound, 1-{4-[(1-isobutyl-1H-imidazo[4,5-c]quinolin-4-yl)amino]phenyl}ethanone 3 was prepared by the amination of 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline 2 with 4-amino acetophenone in n-butanol by using the first method, i.e., nucleophilic substitution reaction. Starting material 4-chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline 2 was in turn synthesized by using literature method [9]. The product 3 was well characterized by using NMR, 13C-NMR, IR and mass spectral data.
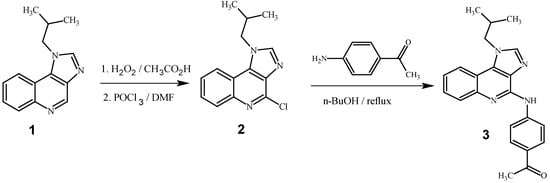
Scheme 1.
Synthesis of 1-{4-[(1-isobutyl-1H-imidazo[4,5-c]quinolin-4-yl)amino]phenyl}ethanone.
Scheme 1.
Synthesis of 1-{4-[(1-isobutyl-1H-imidazo[4,5-c]quinolin-4-yl)amino]phenyl}ethanone.

The 1H NMR spectrum of 1-{4-[(1-isobutyl-1H-imidazo[4,5-c]quinolin-4-yl)amino]phenyl}ethanone 3 showed doublet and septet resonating at δ 0.94 (J = 6.5 Hz) and 2.24 (J = 6.7 Hz) which was assigned to the six methyl protons and one methine proton of isopropyl moiety. A doublet appeared at δ 4.48 (J = 7.4 Hz) was due to the methylene protons. The methyl protons of acetyl group appeared as a singlet at δ 2.53 ppm. The triplet located at δ 7.61 (J = 7.6 Hz) indicated one aromatic H-7 proton and another triplet at δ 7.48 (J = 7.5 Hz) indicated the nearest H-8 aromatic proton of quinoline moeity. A multiplet observed in the region δ 7.90–8.44 ppm for 6 aromatic protons present in the compound. The singlet located at δ 9.68 ppm indicated the presence of one NH proton. Another singlet appeared at δ 8.36 ppm indicated the proton of CH=N group. The IR spectrum showed an absorption band at 1668 cm−1 due to carbonyl stretching. The absorption band at 3,390 cm−1 is due to –NH stretching. Furthermore, the structure was also supported by the mass spectrum of compound 3 which showed a molecular ion peak at m/z 359 (M++1). Elemental analysis and 13C-NMR spectrum also gave satisfactory results for the title compound.
Experimental
Melting point was taken in open capillary tube and was uncorrected. The purity of the compound was confirmed by thin layer chromatography using Merck silica gel 60 F254 coated aluminium plates. IR spectrum was recorded on Shimadzu-FTIR Infrared spectrometer in KBr (νmax in cm−1). 1H-NMR (400 MHz) spectrum was recorded on a Bruker AMX 400 spectrometer, with 5 mm PABBO BB-1H TUBES and 13C-NMR (100 MHz) spectrum was recorded for approximately 0.03 M solutions in DMSO-d6 at 100 MHz with TMS as internal standard. LCMS was obtained using Agilent 1200 series LC and Micromass zQ spectrometer. Elemental analysis was carried out by using VARIO EL-III (Elementar Analysensysteme GmBH).
4-Chloro-1-isobutyl-1H-imidazo[4,5-c]quinoline 2 (1 mmol, 0.259 g) was dissolved in n-butanol (20 mL) and then p-amino acetophenone (1 mmol, 0.135 g) was added. Reaction mixture was refluxed for 6 h. The contents of the flask was concentrated to half of the volume and cooled. The product obtained was filtered, washed with water, dried and recrystallized from ethanol to give the pale yellow colored solid. Yield was 0.302 g (84%).
Melting point: 100–102 °C.
LCMS: m/z = 359 (M++1).
IR (KBr): νmax (cm−1), 3392 (N-H), 2960 (C-H), 1668 (C=O), 1531 (C=C).
1H-NMR (400 MHz, DMSO-d6): δ ppm, 0.94 (d, 6H, CH3×2, J = 6.5 Hz), 2.24 (sep, 1H, CH, J = 6.7 Hz), 2.53 (s, 3H, COCH3), 4.48 (d, 2H, CH2, J = 7.4 Hz), 7.61 (t, 1H, H-7, J = 7.6 Hz), 7.48 (t, 1H, H-8, J = 7.5 Hz), 7.90–8.44 (m, 6H, Ar-H), 8.36 (s, 1H, CH=N), 9.68 (s, 1H, NH).
13C-NMR (100 MHz, DMSO-d6): δ ppm, 19.8 (CH3), 26.8 (COCH3), 28.9 (CH), 54.0 (CH2), 116.2, 118.6, 121.0, 123.9, 127.7, 128.3, 129.2, 129.8, 130.2, 132.6, 144.0, 144.4, 146.1, 147.6 (Ar-C’s), 196.7 (C=O).
Elemental analysis: Calculated for C22H22N4O, C, 73.72%; H, 6.19%; N, 15.63%. Found: C, 73.67%; H, 6.15%; N, 15.57%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
The authors are thankful to Department of Studies in Chemistry, Mangalagangothri, Mangalore University for providing necessary facilities.
References
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles, 2nd ed.; Wiley VCH: Weinheim, Germany, 2003; pp. 316–336. [Google Scholar]
- Uematsu, S.; Akira, S. Toll-like receptors and type I Interferons. J. Biol. Chem. 2007, 282, 15319–15324. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Kaisho, T.; Takeuchi, O. Small-antiviral compounds activate immune cells via the TLR7 MyD88- dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.A. Imiquimod and the imidazoquinolones: Mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 2002, 27, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.J.; Testerman, T.L.; Miller, R.L.; Weeks, C.E.; Tomai, M.A. Cytokine induction in mice by the immunomodulator imiquimod. J. Leukoc. Biol. 1994, 55, 234–240. [Google Scholar] [PubMed]
- Miller, R.L.; Gerster, J.F.; Owens, M.L.; Slade, H.B.; Tomai, M.A. Imiquimod applied topically: A novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 1999, 21, 1–14. [Google Scholar] [CrossRef]
- Philip, J.M.; Galen, V.; Peter, N.; Wijngaarden, I.V.; Adriaan, P.I.; Willem, S. 1H-Imidazo[4,5-c]quinolin-4-amines: Novel non-xanthine adenosine antagonists. J. Med. Chem. 1991, 34, 1202–1206. [Google Scholar]
- Merli, V.; Mantovani, S.; Bianchi, S. Preparation of 1H-imidazo[4,5-c]quinolin-4-amines via 1H-imidazo[4,5-c]quinolin-4-phthalimide intermediates. U.S. Patent US2006/7153967(B2), 26 December 2006. [Google Scholar]
- Colombo, L.; Mariotti, E.; Allegrini, P.; Castaldi, G. Process for the preparation of Imiquimod and intermediates thereof. U.S. Patent US2008/0161573(A1), 03 July 2008. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
