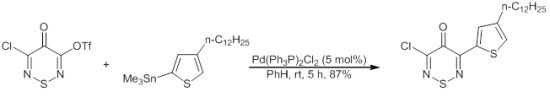
3-Chloro-5-(4-dodecylthiophen-2-yl)-4H-1,2,6-thiadiazin-4-one
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Geevers, J.; Trompen, W.P. Synthesis and Reactions of 3,5-Dichloro-4H-1,2,6-Thiadiazin-4-one. Recl. Trav. Chim. Pays-Bas 1974, 93, 270–272. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Chem. Commun. 2000, 303–304. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Cyclisation chemistry of 4H-1,2,6-thiadiazines. J. Chem. Soc. Perkin Trans. 1 2000, 2601–2607. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A.; Rikkou, M.D. The synthesis of pyrrolo[2,3-c][1,2,6]thiadiazine-5-carbonitriles from (4H-1,2,6-thiadiazin-4-ylidene)malononitriles. Tetrahedron 2010, 66, 1817–1820. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Chemistry of 4-dicyanomethyleno-1,2,6-thiadiazines. J. Chem. Soc. Perkin Trans. 1 2000, 1081–1088. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Palladium Catalyzed C-C Coupling Reactions of 3,5-Dichloro-4H-1,2,6-thiadiazin-4-one. Org. Lett. 2011, 13, 3466–3469. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Kizas, C.; Koutentis, P.A. Selective Stille Coupling Reactions of 3-Chloro-5-halo(pseudohalo)-4H-1,2,6-thiadiazin-4-ones. Org. Lett. 2011, 13, 5886–5889. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, H.A.; Koutentis, P.A. Modification of C-4 Position of 3,5-Disubstituted 4H-1,2,6-Thiadiazin-4-ones. Tetrahedron 2012, 68, 2590–2597. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Koutentis, P.A. Synthesis of Asymmetric 3,5-Diaryl-4H-1,2,6-Thiadiazin-4-ones via Suzuki-Miyaura and Stille Coupling Reactions. Tetrahedron 2012, 68, 7380–7385. [Google Scholar] [CrossRef]
- Mishra, S.P.; Palai, A.K.; Srivastava, R.; Kamalasanan, M.N.; Patri, M. Dithieno[3,2-b:2′,3′-d]pyrrole–alkylthiophene–benzo[c][1,2,5]thiadiazole-based highly stable and low band gap polymers for polymer light-emitting diodes. J. Polym. Sci. A Polym. Chem. 2009, 47, 6514–6525. [Google Scholar] [CrossRef]



© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ioannidou, H.A.; Koutentis, P.A. 3-Chloro-5-(4-dodecylthiophen-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank 2012, 2012, M782. https://doi.org/10.3390/M782
Ioannidou HA, Koutentis PA. 3-Chloro-5-(4-dodecylthiophen-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank. 2012; 2012(4):M782. https://doi.org/10.3390/M782
Chicago/Turabian StyleIoannidou, Heraklidia A., and Panayiotis A. Koutentis. 2012. "3-Chloro-5-(4-dodecylthiophen-2-yl)-4H-1,2,6-thiadiazin-4-one" Molbank 2012, no. 4: M782. https://doi.org/10.3390/M782
APA StyleIoannidou, H. A., & Koutentis, P. A. (2012). 3-Chloro-5-(4-dodecylthiophen-2-yl)-4H-1,2,6-thiadiazin-4-one. Molbank, 2012(4), M782. https://doi.org/10.3390/M782