Abstract
An aziridine-containing side chain is attached to an oxonaphthalene-annelated pyrrole in expectation of DNA alkylating properties. The cytotoxicity is evaluated against two cell lines, KB-31 and KB-8511, respectively.
Introduction
Chlorambucil and melphalan are chemotherapy drugs belonging to the class of nitrogen mustard alkylating agents. Both compounds are believed to exert their antitumor effects by cross-linking DNA via aziridinium cation intermediates arising from the bis(2-chloroethyl)amine moiety [1]. In continuation of our department’s previous studies in the field of antitumor agents [2,3,4,5,6,7,8,9,10], we are reporting in this paper the synthesis of the oxonaphthalene-annelated pyrrole 2 with an attached side chain containing an aziridine group. The rationale is that the three-membered aziridine ring is structurally analogous to the ammonium-intermediate formed from the nitrogen mustards. The aziridine moiety is not charged and the reactivity results from the strain on the three-member ring structure [11]. Recent studies with aziridine substituted quinones showed promising results against breast cancer tumor cells [12,13,14,15,16]. The cytotoxic activity of 2 was evaluated.
Results and Discussion
Reaction of 1 [17] with t-BuPh2Si-protected hydroxyethyl bromide [18] with NaH in THF afforded the N-alkylated product. The following deprotection with tetrabutylammonium fluoride [19] furnished the alcohol which was treated with methansulfonic chloride [20]. The resulting mesylate was converted via reaction with aziridine into the target compound 2 (Scheme 1). The biological activity of 2 was tested against two cancer cell lines, KB-31 and KB-8511, respectively. KB-31 is a drug-sensitive human epidermoid cell line, whereas KB-8511 is a multi-drug resistant subline, typically overexpressing P-glycoprotein. The IC50[μM] values of 2 are >10.000 (KB-31) and 8.680 (KB-8511), respectively (3 days incubation time; staining with 0.05% methylene blue; optical density measured at 665 nm; for further experimental details, see [21,22]).
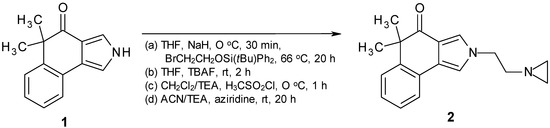
Scheme 1.
Synthesis of target compound 2.
Experimental
2-[2-(Aziridin-1-yl)ethyl]-5,5-dimethyl-2,5-dihydro-4H-benzo[e]isoindol-4-one (2)
(a) To a solution of 0.3 g (12.38 mmol) NaH (60% in mineral oil, washed twice with hexane) in 20 mL of dry THF was added dropwise under argon a solution of 1 [8] (2.61 g, 12.38 mmol) in 20 mL of dry THF. After stirring for 0.5 h at 0 °C at room temperature, a solution of 6.74 g (18.58 mmol) of 2-(bromoethoxy)(tert-butyl)diphenylsilane in 30 mL of dry THF was added. After stirring for 20 h under reflux the reaction mixture was treated with a saturated aqueous solution of ammonium chloride and extracted with ether. The organic phase was dried (Na2SO4) and concentrated. Yield 3.34 g (55%) of colorless crystals (m.p. 117–118 °C, TLC, silica gel, light petroleum/ethyl acetate 70/30).
(b) The resulting product from (a) (3.34 g, 6.78 mmol) was dissolved under argon in a mixture of 40 mL of dry THF and 13.5 mL of a 1M solution of TBAF in THF and stirred for 2 h at room temperature. Subsequently after addition of H2O the resulting mixture was extracted with ether. The ether extract was dried (Na2SO4) and concentrated. Yield 1.23 g (71%) of colorless crystals (m.p. 112–113 °C, TLC, silica gel, ethyl acetate/light petroleum 80/20).
(c) The obtained product from (b) (1.23 g, 4.88 mmol) was dissolved under argon in a mixture of 1.0 mL (7.39 mmol) of dry TEA and 16 mL of dry CH2Cl2. Afterwards 0.46 mL (5.89 mmol) of freshly distilled methanesulfonic chloride under argon was added dropwise and the resulting reaction mixture was stirred for 1 h at 0 °C. Subsequently the reaction mixture was extracted with CH2Cl2, dried (Na2SO4) and concentrated. Yield: 1.56 g (96%) of colorless crystals (m.p. 122–123 °C, TLC, silica gel, ethyl acetate/light petroleum 80/20).
(d) The resulting crude product from (c) (1.56 g, 4.67 mmol) was dissolved under argon in a dry mixture of acetonitrile/triethyl amine (18 mL, 1:1) and treated with 9.69 mL (18.7 mmol) of aziridine. After stirring for 20 h at room temperature the reaction mixture was diluted with a mixture of CH2Cl2/EtOH (9/1) and subsequently filtered by use of 125 g of silica gel. Evaporation furnished 1.27 g of crude product which was purified by column chromatography (silica gel, ethyl acetate/triethylamine 95/5) to afford 0.29 g (22%) of colorless crystals of 2. M.p. 96–98 °C (ethyl acetate). IR (KBr): 3350, 2950, 1643, 1523, 1207, 1160 cm−1. MS (EI, 70 eV) m/z: 280 (M+, 10%), 224 (M+-56, 1), 88 (17), 73 (20), 70 (67), 61 (81), 56 (59), 45 (100). 1H-NMR (CDCl3, 200 MHz) δ = 7.56 (m, 1H, 9-H), 7.46 (m, 1H, 6-H), 7.41 (d, J = 2.0 Hz, 1H, 3-H), 7.21 (m, 2H, 7-H, 8-H), 7.07 (d, J = 2.0 Hz, 1H, 1-H), 4.15 (t, J = 5.9 Hz, 2H, 1'-H), 2.60 (t, J = 5.9 Hz, 2H, 2'-H) 1.72 (m, 2H, aziridine-H), 1.51 (s, 6H, (CH3)2), 1.03 (m, 2H, aziridine-H). 13C-NMR (CDCl3, 50 MHz) δ = 198.4 (C-4), 144.1 (C-5a), 127.0 (C-6), 126.9 (C-9a), 126.6 (C-7), 126.2 (C-8), 125.1 (C-9b), 123.5 (C-3), 122.6 (C-9), 118.5 (C-3a), 115.5 (C-1), 61.6 (C-2'), 50.8 (C-1'), 47.6 (C-5), 28.1 ((CH3)2), 27.0 (aziridine-CH2). HRMS calc. for C18H20N2O: 280.1576. Found: 280.1569.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
We are indebted to Novartis AG (Vienna, Austria) for the evaluation of the cytotoxic activity.
References and Notes
- Montgomery, J.A. Agents That React with DNA. In Cancer Chemotherapeutic Agents, ACS Professional Reference Book; Foye, W.O., Ed.; American Chemical Society: Washington, DC, USA, 1995; pp. 111–121. [Google Scholar]
- Shanab, K.; Schirmer, E.; Wulz, E.; Weissenbacher, B.; Lassnig, S.; Slanz, R.; Fösleitner, G.; Holzer, W.; Spreitzer, H.; Schmidt, P.; et al. Synthesis and antiproliferative activity of new cytotoxic azanaphthoquinone pyrrolo-annelated derivatives: Part II. Bioorg. Med. Chem. Lett. 2011, 21, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Shanab, K.; Schirmer, E.; Knafl, H.; Wulz, E.; Holzer, W.; Spreitzer, H.; Schmidt, P.; Aicher, B.; Mueller, G.; Guenther, E. Synthesis and antiproliferative activity of new cytotoxic azanaphthoquinone pyrrolo-annelated derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 3950–3952. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, H.; Puschmann, C. 1-[4-[Bis(2-chloroethyl)amino]benzyl]-5,5-dimethyl-2,5-dihydro-4H-benzo[e]isoindol-4-one (Cytotoxic Oxonaphthalene-Pyrroles, Part II). Molbank 2010, 2010, M654. [Google Scholar] [CrossRef]
- Spreitzer, H.; Puschmann, C. 2-[4-[Bis(2-chloroethyl)amino]benzyl]-5,5-dimethyl-2,5-dihydro-4H-benzo[e]isoindol-4-one (Cytotoxic Oxonaphthalene-Pyrroles, Part I). Molbank 2010, 2010, M651. [Google Scholar] [CrossRef]
- Pongprom, N.; Bachitsch, H.; Bauchinger, A.; Ettefagh, H.; Haider, T.; Hofer, M.; Knafl, H.; Slanz, R.; Waismayer, M.; Wieser, F.; et al. Synthesis of new Benzo[f]isoindole-4,9-diones as anticancer compounds. Monatsh. Chem. 2010, 141, 53–52. [Google Scholar] [CrossRef]
- Spreitzer, H.; Puschmann, C. Regioselective alkylation of an oxonaphthalene-annelated pyrrol system. Molbank 2009, 2009, M619. [Google Scholar] [CrossRef]
- Pongprom, N.; Müller, G.; Schmidt, P.; Holzer, W.; Spreitzer, H. Synthesis of anticancer compounds, III (Bioorg Med Chem Lett 17, 6091, 2007), carbinol derivatives of azanaphthoquinone annelated pyrrols. Monatsh. Chem. 2009, 140, 309–313. [Google Scholar] [CrossRef]
- Shanab, K.; Pongprom, N.; Wulz, E.; Holzer, W.; Spreitzer, H.; Schmidt, P.; Aicher, B.; Müller, G.; Günther, E. Synthesis and biological evaluation of novel cytotoxic azanaphthoquinone annelated pyrrolo oximes. Bioorg. Med. Chem. Lett. 2007, 17, 6091–6095. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, H.; Puschmann, C. Synthesis of Anticancer Compounds, I, “Dual function” antitumor agents based on bioreduction and DNA-alkylation. Monatsh. Chem. 2007, 138, 517–522. [Google Scholar] [CrossRef]
- Pratt, W.B.; Ruddon, R.W.; Ensminger, W.D.; Maybaum, J. Covalent DNA Binding Drugs. In The Anticancer Drugs, 2nd ed.; Oxford University Press: New York, NY, USA, 1994; pp. 108–154. [Google Scholar]
- Huang, C.H.; Kuo, H.S.; Liu, J.W.; Lin, Y.L. Synthesis and Antitumor Evaluation of Novel Bis-Triaziquone Derivatives. Molecules 2009, 14, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Su, Y.T.; Chen, B.H. A study on inhibition mechanism of breast cancer cells by bis-type triziquone. Eur. J. Pharmacol. 2010, 637, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fahey, K.; O’Donovan, L.; Carr, M.; Carty, M.P.; Aldabbah, F. The influence of the aziridinyl substituent of benzimidazoles and benzimidazolequinones on toxicity towards normal and Fanconi anaemia cells. Europ. J. Med. Chem. 2010, 45, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, L.; Carty, M.P.; Aldabbagh, F. First synthesis of N-[(aziridin-2-yl)methyl]benzimidazolequinone and analysis of toxicity towards normal and Fanconi anaemia cells. Chem. Commun. 2008, 5592–5594. [Google Scholar]
- Bonham, S.; O’Donovan, L.; Cary, M.P.; Aldabbagh, F. First synthesis of an aziridinyl fused pyrrolo[1,2-a]benzimidazole and toxicity evaluation towards normal and breast cancer cell lines. Org. Biomol. Chem. 2011, 9, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, H.; Holzer, W.; Puschmann, C.; Pichler, A.; Kogard, A.; Tschetschkowitsch, K.; Heinze, T.; Bauer, S.; Shabaz, N. Synthesis and NMR-investigation of annelated pyrrole derivatives. Heterocycles 1997, 45, 1989–1997. [Google Scholar] [CrossRef]
- Rudisill, D.E.; Stille, J.K. Palladium-catalyzed synthesis of 2-substituted indoles. J. Org. Chem. 1989, 54, 5856–5866. [Google Scholar] [CrossRef]
- Groth, U.; Halfbrodt, W.; Koehler, T.; Kreye, P. Synthesis of (±)-chokol A by a tandem Michael-addition/Dieckmann cyclization. Liebigs Ann. Chem. 1994, 9, 885–890. [Google Scholar] [CrossRef]
- Artis, D.R.; Cho, I.S.; Jaime-Figueroa, S.; Muchowski, J.M. Oxidative Radical Cyclization of (ω-Iodoalkyl)indoles and Pyrroles. Synthesis of (−)-Monomorine and Three Diastereomers. J. Org. Chem. 1994, 59, 2456–2466. [Google Scholar] [CrossRef]
- Meyer, T.; Regenass, U.; Fabbro, D; Alteri, E.; Rösel, J.; Müller, M.; Caravatti, G.; Matter, A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer 1989, 43, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Scarpelli, R.; Bollbuck, B.; Werschkun, B.; Pereira, M.M.; Wartmann, M.; Altmann, K.H.; Zaharevitz, D.; Guscio, R.; Giannakakou, P. Chemical synthesis and biological properties of pyridine epothilones. Chem. Biol. 2000, 7, 593–599. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).