(Z)-N-(7-Cyano-9,9,15,15-tetramethyl-9,10,11,13,14,15-hexahydro-6H-benzo[4'',5'']imidazo[1'',2'':1',2']pyrido[3',4':5, 6]pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-6-ylidene)pent-4-ynamide
Abstract
:Introduction
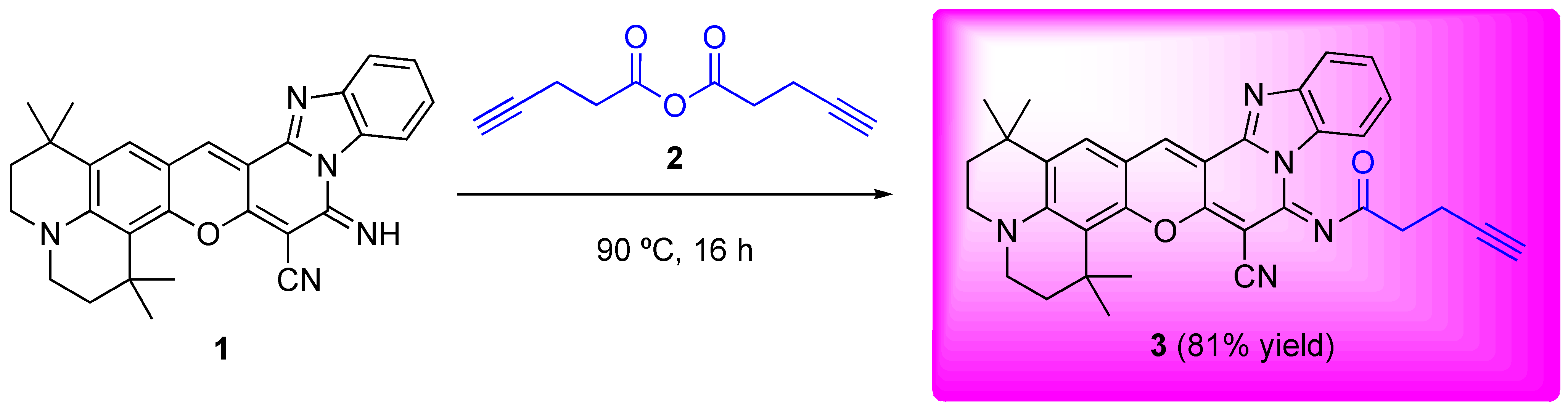
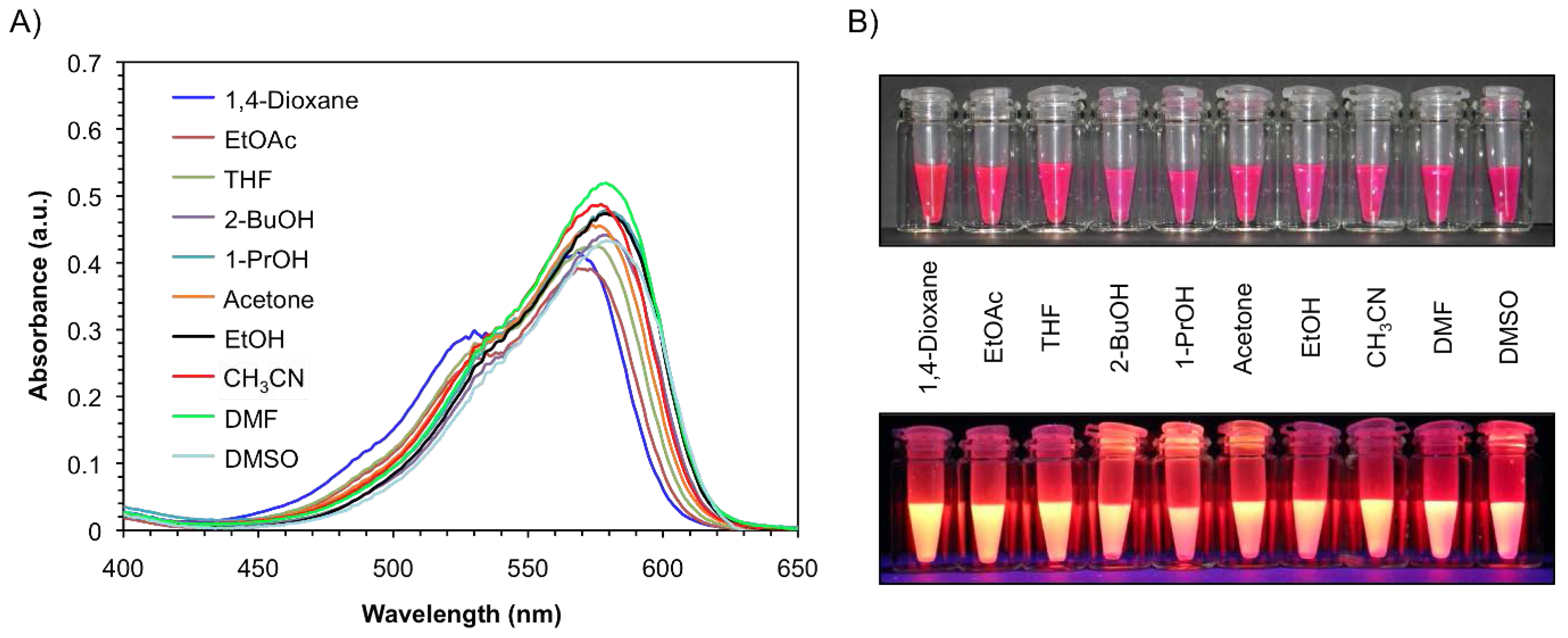
Results and Discussion
Experimental
General
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References and Notes
- Krasovitskii, B.M.; Bolotin, B.M. Organic Luminescent Materials; Wiley-VCH: Weinheim, Germany, 1988. [Google Scholar]
- Christie, R.M. Fluorescent dyes. Rev. Prog. Color. Relat. Top. 1993, 23, 1–18. [Google Scholar] [CrossRef] and references therein.
- O’Kennedy, R. Coumarins; John Wiley: New York, NY, USA, 1997. [Google Scholar]
- Lewis, P.A. The Pigment Handbook, 2nd ed.; John Wiley: New York, NY, USA, 1988; Volume 1, pp. 859–872. [Google Scholar]
- Miyata, S. Organic Electroluminescent Materials and Devices, 1st ed.; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Kim, S.-H. Functional Dyes, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Singapore, 2012. [Google Scholar]
- Patai, S. Chemistry of Triple-Bonded Functional Groups; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Acetylene Chemistry: Chemistry, Biology, and Material Science; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloadditions. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed] and references therein.
- BASF German P 2 415 661 (1975).
- Mazzei, M.; Ermili, A.; Balbi, A.; di Braccio, M.; Schiantarelli, P.; Cadel, S. Chemistry and pharmacology of pyran derivatives. XVII. Synthesis of 2-(dialkylamino)-5-hydroxychromones and their transformation to derivatives of 2H-pyran[4,3,2-de]-1-benzopyran. Farmaco Sci. 1986, 8, 611–621. [Google Scholar]
- See Supplementary Files for detailed information.
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Malkoch, M.; Schleicher, K.; Drockenmuller, E.; Hawker, C.J.; Russell, T.P.; Wu, P.; Fokin, V.V. Structurally Diverse Dendritic Libraries: A Highly Efficient Functionalization Approach Using Click Chemistry. Macromolecules 2005, 9, 3663–3678. [Google Scholar] [CrossRef]
- If necessary, the amount of pent-4-ynoic anhydride can be reduced (6-fold) using DMF as solvent over a period of 12 h.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bachl, J.; Wolfbeis, O.; Cativiela, C.; Díaz, D.D. (Z)-N-(7-Cyano-9,9,15,15-tetramethyl-9,10,11,13,14,15-hexahydro-6H-benzo[4'',5'']imidazo[1'',2'':1',2']pyrido[3',4':5, 6]pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-6-ylidene)pent-4-ynamide. Molbank 2012, 2012, M783. https://doi.org/10.3390/M783
Bachl J, Wolfbeis O, Cativiela C, Díaz DD. (Z)-N-(7-Cyano-9,9,15,15-tetramethyl-9,10,11,13,14,15-hexahydro-6H-benzo[4'',5'']imidazo[1'',2'':1',2']pyrido[3',4':5, 6]pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-6-ylidene)pent-4-ynamide. Molbank. 2012; 2012(4):M783. https://doi.org/10.3390/M783
Chicago/Turabian StyleBachl, Jürgen, Otto Wolfbeis, Carlos Cativiela, and David D. Díaz. 2012. "(Z)-N-(7-Cyano-9,9,15,15-tetramethyl-9,10,11,13,14,15-hexahydro-6H-benzo[4'',5'']imidazo[1'',2'':1',2']pyrido[3',4':5, 6]pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-6-ylidene)pent-4-ynamide" Molbank 2012, no. 4: M783. https://doi.org/10.3390/M783
APA StyleBachl, J., Wolfbeis, O., Cativiela, C., & Díaz, D. D. (2012). (Z)-N-(7-Cyano-9,9,15,15-tetramethyl-9,10,11,13,14,15-hexahydro-6H-benzo[4'',5'']imidazo[1'',2'':1',2']pyrido[3',4':5, 6]pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-6-ylidene)pent-4-ynamide. Molbank, 2012(4), M783. https://doi.org/10.3390/M783





