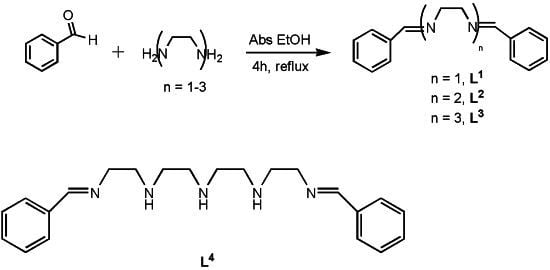
N1-Benzylidene-N2-(2-((2-((2-(benzylideneamino)ethyl)amino) ethyl)amino)ethyl)ethane-1,2-diamine
Abstract
:Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vujcic, S.; Diegelman, P.; Bacchi, C.J.; Kramer, D.L.; Porter, C.W. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem. J. 2002, 367, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V. Polyamine analogues as anticancer drugs. Biochem. Soc. Trans. 2003, 31, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Bachrach, U. (Eds.) Special issue: Polyamines and their Analogs in Cancer and other Diseases. Amino Acids 2007, 33, 175–187.
- Sonawane, N.D.; Szoka, F.C., Jr.; Verkman, A.S. Chloride Accumulation and Swelling in Endosomes Enhances DNA Transfer by Polyamine-DNA Polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [PubMed]
- Albelda, M.T.; Díaz, P.; García-España, E.; Lima, J.C.; Lodeiro, C.; de Melo, J.S.; Parola, A.J.; Pina, F.; Soriano, C. Switching from intramolecular energy transfer to intramolecular electron transfer by the action of pH and Zn(II) coordination. Chem. Phys. Lett. 2002, 353, 63–68. [Google Scholar] [CrossRef]
- De Melo, J.S.; Pina, J.; Pina, F.; Lodeiro, C.; Parola, A.J.; Albelda, M.T.; Clares, M.P.; García-España, E.; Soriano, C. Energetics and dynamics of naphathalene polyaminic derivatives. Influence of structural design in the balance static vs dynamic excimer formation. J. Phys. Chem. A 2003, 107, 11307–11318. [Google Scholar] [CrossRef]
- Alarcón, J.; Aucejo, R.; Albelda, M.T.; Alves, S.; Clares, P.; García-España, E.; Lodeiro, C.; Marchin, K.L.; Parola, A.J.; Pina, F.; et al. Fluorescent Type II Materials from Naphthylmethyl Polyamine Precursors. Supramol. Chem. 2004, 16, 573–580. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Danesi, A.; Faggi, E.; Giorgi, C.; Lodeiro, C.; Oliveira, E.; Pina, F.; Valtancoli, B. Interaction of polyamine macrocycles with Zn(II) and ATP in aqueos solution. Binary and Ternary systems. A potentiometric, NMR and fluorescence emission studies. Inorg. Chim. Acta 2008, 361, 3410–3419. [Google Scholar] [CrossRef]
- Fernandez, L.; Boucher, M.; Fernández-Lodeiro, J.; Oliveira, E.; Núñez, C.; Santos, H.M.; Capelo, J.L.; Nieto-Faza, O.; Bértolo, E.; Lodeiro, C. Exploiting anionic and cationic interactions with a new emissive imine-based β-naphtol molecular probe. Inorg. Chem. Commun. 2009, 12, 905–912. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, J.; Núñez, C.; Carreira, R.; Santos, H.M.; Silva-López, C.; Mejuto, J.C.; Capelo, J.L.; Lodeiro, C. Novel versatile imine-enamine chemosensor based on 6-nitro-4-oxo-4H-chromene for ion detection in solution, solid and gas-phase: Synthesis, emission, computational and MALDI-TOF-Ms studies. Tetrahedron 2011, 67, 326–333. [Google Scholar] [CrossRef]
- Bernardo, M.A.; Gurrero, J.A.; García-España, E.; Luis, S.V.; Llinares, J.M.; Pina, F.; Ramírez, J.A.; Soriano, C. Thermodynamic, NMR and photochemical study on the acid-base behaviour or N,N'-dibenzylated polyamines and on their interactions with hexacyanocobaltate(III). J. Chem. Soc. Perkin Trans 2 1996, 2335–2342. [Google Scholar] [CrossRef]
- The smaller parent compounds derived from 1,2-ethanediamine (L1), diethylenetriamine (L2), and triethylenetetramine (L3) were obtained by a similar methodology, using 0.038, 0.063 and 0.089 g of diamine, respectively. Compound L1: N1,N2-Dibenzylideneethane-1,2-diamine; Yield: 121 mg (84%); ESI-MS: m/z (rel. int%): 237.13 (100) ([M+H]+); 1H NMR (CDCl3): δ = 8.1 (s, 2H, N=C–H); 7.8 (m, 4H, C-Har); 7.2 (m, 6H, C-Har); 3.8 (s, 4H, CH2) ppm; IR (cm−1): 1647 (C=N, Imine), 1599, 1498 (C=C, Ar); Elemental analysis: Calcd for C16H16N2: C, 81.32; H, 6.82; N, 11.85. Found: C, 80.87; H, 7.02; N,12.05. Compound L2: N1-Benzylidene-N2-(2-(benzylideneamino)¬ethyl)ethane-1,2-diamine; Yield: 103 mg (71%); ESI-MS: m/z (rel. int%): 279.17 (100) ([M+H]+); 1H-NMR (CDCl3): δ = 8.2 (s, 2H, N=C–H); 7.8–7.6 (m, 4H, C-Har); 7.4–7.2 (m, 6H, C-Har); 3.8 (m, 4H, CH2); 2.9 (m, 4H, CH2) ppm; IR (cm−1): 1649 (C=N, Imine), 1586, 1491 (C=C, Ar); Elemental analysis: Calcd for C18H21N3: C, 77.38; H, 7.58; N, 15.04. Found: C, 77.16; H, 8.03; N, 15.34. Compound L3: N1,N1′-(Ethane-1,2-diyl)bis(N2-benzylideneethane-1,2-diamine); Yield: 132 mg (89%); ESI-MS: m/z (rel. int%): 323.22 (100) ([M+H]+); 1H-NMR (CDCl3): δ = 8.1 (s, 2H, N=C–H); 7.7–7.5 (m, 4H, C-Har); 7.4–7.1 (m, 6H, C-Har); 3.7–3.4 (m, 2H, CH2); 2.9–2.1 (m, 8H, CH2) ppm; IR (cm−1): 1656 (C=N, Imine), 1576, 1499 (C=C, Ar); Elemental analysis: Calcd for C20H26N4: C, 74.50; H, 8.13; N, 17.38. Found: C, 74.78; H, 8.16; N, 17.49.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernández-Lodeiro, J.; Núñez, C.; Bértolo, E.; Capelo, J.L.; Lodeiro, C. N1-Benzylidene-N2-(2-((2-((2-(benzylideneamino)ethyl)amino) ethyl)amino)ethyl)ethane-1,2-diamine. Molbank 2012, 2012, M779. https://doi.org/10.3390/M779
Fernández-Lodeiro J, Núñez C, Bértolo E, Capelo JL, Lodeiro C. N1-Benzylidene-N2-(2-((2-((2-(benzylideneamino)ethyl)amino) ethyl)amino)ethyl)ethane-1,2-diamine. Molbank. 2012; 2012(4):M779. https://doi.org/10.3390/M779
Chicago/Turabian StyleFernández-Lodeiro, Javier, Cristina Núñez, Emilia Bértolo, José Luis Capelo, and Carlos Lodeiro. 2012. "N1-Benzylidene-N2-(2-((2-((2-(benzylideneamino)ethyl)amino) ethyl)amino)ethyl)ethane-1,2-diamine" Molbank 2012, no. 4: M779. https://doi.org/10.3390/M779
APA StyleFernández-Lodeiro, J., Núñez, C., Bértolo, E., Capelo, J. L., & Lodeiro, C. (2012). N1-Benzylidene-N2-(2-((2-((2-(benzylideneamino)ethyl)amino) ethyl)amino)ethyl)ethane-1,2-diamine. Molbank, 2012(4), M779. https://doi.org/10.3390/M779