Abstract
The reaction of 1-phenyl-3-trifluoromethyl-2-pyrazolin-5-one and 2-chloro¬benzaldehyde leads to the title compound, which results from addition of a second pyrazolone unit to the primarily formed 1:1 condensation product. Detailed spectroscopic data (1H NMR, 13C NMR, 19F NMR, IR, MS) are presented.
In the course of a synthetic program dedicated to the functionalization of 1-phenyl-3-trifluoromethyl-2-pyrazolin-5-one (1) [1] we were interested in compound 2, which was considered as precursor in the synthesis of the chromeno[2,3-c]pyrazol-4(1H)-one 3 (Scheme 1). The synthesis of compounds similar to 2 has been described by Sobahi [2] and by Zohdi [3]: heating equimolar amounts of 1 and differently substituted benzaldehydes to 160 °C without a solvent resulted in the formation of the corresponding 1:1 condensation products [2]. However, when we applied such conditions (160 °C) in the reaction of 1 and 2-chlorobenzaldehyde we only obtained a product which – considering its characteristic spectroscopic data – turned out to be the 2:1 adduct 4b. Obviously, the primarily formed 1:1 condensation product 2 instantly reacts with a second pyrazolone unit to afford dimer 4b. Also the application of other reaction conditions (lower temperature, excess aldehyde, refluxing EtOH with catalytic amounts of piperidine) afforded 4b as well. The formation of dimeric moieties similar to 4 starting from 1 and substituted benzaldehydes upon heating in aqueous medium without a catalyst was recently reported by Yao and coworkers [4]. The structure of these products was unequivocally proved by X-ray structure analysis [4].
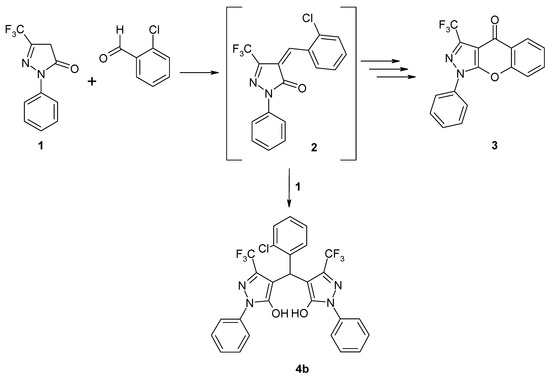
Scheme 1.
When we furthermore reacted 1 also with benzaldehyde and 2,4-dichlorobenzaldehyde, respectively, we obtained the corresponding dimeric compounds (4a, 4c) as well (Figure 1). Melting points and 1H-NMR spectroscopic data of the latter were in full agreement with those given by Yao [4].
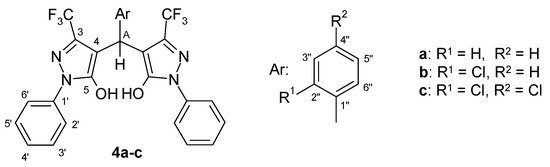
Figure 1.
Numbering of atoms in the representation of NMR spectra.
The NMR spectra of compounds 4 exhibit the signals of the characteristic, central C(sp3)-H system (δ H: 5.22–5.68 ppm; δ C: 30.7–32.8 ppm; 1J: 124.5–128.0 Hz). For comparison reasons, the NMR spectroscopic data of the hitherto unknown title compound 4b are presented together with those of known congeners 4a and 4c in Table 1 and Table 2.

Table 1.
1H-NMR and 19F-NMR chemical shifts of compounds 4a-c (δ, ppm, in DMSO-d6).

Table 2.
13C-NMR data (δ, ppm; J in Hz; in DMSO-d6).
Experimental
Melting points were determined on a Reichert–Kofler hot-stage microscope and are uncorrected. Mass spectra were obtained on a Shimadzu QP 1000 instrument (EI, 70 eV). IR spectrum: Perkin-Elmer FTIR Spectrum 1000 instrument (KBr-disc). The elemental analysis was performed at the Microanalytical Laboratory, University of Vienna. 1H and 13C NMR spectra were recorded on a Varian UnityPlus 300 spectrometer at 28 °C (299.95 MHz for 1H, 75.43 MHz for 13C). The centre of the solvent signal was used as an internal standard which was related to TMS with δ = 2.49 ppm (1H in DMSO-d6) and δ = 39.5 ppm (13C in DMSO- d6). The digital resolutions were 0.2 Hz/data point in the 1H and 0.4 Hz/data point in the 1H-coupled 13C-NMR spectra (gated decoupling). 19F NMR spectra (470.56 MHz) were obtained on a Bruker Avance 500 instrument with a ‘directly’ detecting broadband observe probe (BBFO) and were referenced using the absolute frequency scale (Ξ ratio). Unambiguous assignment of signals was accomplished using standard NMR techniques such as HSQC, HMBC, NOE-difference and fully 1H-coupled 13C-NMR (gated decoupling) [5].
4,4’-[(2-Chlorophenyl)methylene]bis[1-phenyl-3-(trifluoromethyl)-1H-pyrazol-5-ol] (4b)
Under N2 atmosphere, a mixture of 1-phenyl-3-(trifluoromethyl)-2-pyrazolin-5-one (1) (500 mg, 2.19 mmol) and 2-chlorobenzaldehyde (616 mg, 4.38 mmol) was heated at 120-130 °C with stirring for 15 min. After cooling to room temperature, 9 mL of light petroleum and 1 mL of ethyl acetate were added and the mixture was refluxed for 15 min, then allowed to cool slowly to ambient temperature. The precipitated solid was filtered off, washed with the same solvent mixture and dried to afford 453 mg (72 %) of the title compound as colorless crystals, mp 202-204 °C.
IR (KBr) ν (cm-1): 3445 (OH).
MS (EI, 70 eV): (m/z, %) M+ not found, 350/352 (5/2), 316 (20), 315 (100), 228 (32), 105 (16), 91 (13), 77 (95), 51(29).
Anal. Calcd for C27H17ClF6N4O2 • ⅓ H2O: C, 55.53%; H, 3.04%; N, 9.58%. Found: C, 55.53%; H, 2.72%; N, 9.50%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
Dr. Changbin Guo thanks the Eurasia-Pacific Uninet Program for providing a scholarship.
References and Notes
- Bieringer, S.; Holzer, W. 4-Acyl-5-hydroxy-1-phenyl-3-trifluoromethylpyrazoles: Synthesis and NMR Spectral Investigations. Heterocycles 2006, 68, 1825–1836. [Google Scholar]
- Sobahi, T.R. Thermal condensation of 3-trifluoromethyl- and 3-amino-1-phenyl-2-pyrazolin-5-ones with aromatic aldehydes: Synthesis of 4-arylidenepyrazolones and pyrazolopyranopyrazoles. Indian J. Chem, Sect. B 2006, 45, 1315–1318. [Google Scholar] [CrossRef]
- Zohdi, H.F.; Elghandour, A.H.H.; Rateb, N.M.; Sallam, M.M.M. Reactions with 5-trifluoromethyl-2,4-dihydropyrazol-3-one derivatives: a new route for the synthesis of fluorinated polyfunctionally substituted pyrazole and pyrano[2,3-c]pyrazole derivatives. J. Chem. Res. Synop. 1992, 396–397. [Google Scholar] [CrossRef]
- Yao, C.S.; Yu, C.X.; Tu, S.J.; Shi, D.Q.; Wang, X.S.; Zhu, Y.Q.; Yang, H.Z. The synthesis of 4,4’-arylmethylene-bis(3-trifluoromethyl)-1-phenyl-1H-pyrazol-5-ol) in aqueous media without catalyst. J. Fluorine Chem. 2007, 128, 105–109. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.O.; Berger, S. 150 and More Basic NMR Experiments, 2nd expanded ed.; Wiley-VCH: Weinheim, New York, 1998. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).