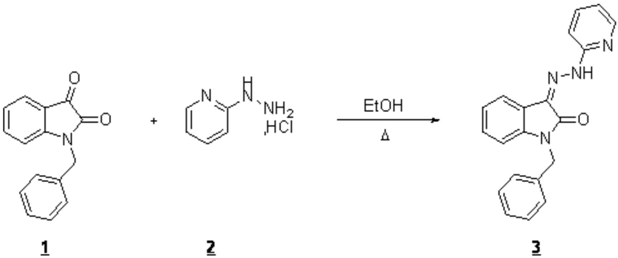
1-benzyl-3-(2-(pyridin-2-yl)hydrazono)indolin-2-one
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Verma, M.; Pandeya, S.N.; Singh, K.N.; Stables, J.P. Acta Pharm. 2004, 54, 49. [PubMed]
- Guengerich, F.P.; Sorrells, J.L.; Schmitt, S.; Krauser, J.A.; Aryal, P.; Meijer, L. J. Med. Chem. 2004, 47, 3236. [PubMed]
- Pirrung, M.C.; Pansare, S.V.; Sarma, K.D.; Keith, K.A.; Kern, E.R. J. Med. Chem. 2005, 48, 3045. [PubMed]
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Rachid, B.; Joly, N.; Lequart, V.; Martin, P.; Massoui, M.; Essassi, E.M. 1-benzyl-3-(2-(pyridin-2-yl)hydrazono)indolin-2-one. Molbank 2007, 2007, M535. https://doi.org/10.3390/M535
Rachid B, Joly N, Lequart V, Martin P, Massoui M, Essassi EM. 1-benzyl-3-(2-(pyridin-2-yl)hydrazono)indolin-2-one. Molbank. 2007; 2007(2):M535. https://doi.org/10.3390/M535
Chicago/Turabian StyleRachid, Bouhfid, Nicolas Joly, Vincent Lequart, Patrick Martin, Mohamed Massoui, and El Mokhtar Essassi. 2007. "1-benzyl-3-(2-(pyridin-2-yl)hydrazono)indolin-2-one" Molbank 2007, no. 2: M535. https://doi.org/10.3390/M535
APA StyleRachid, B., Joly, N., Lequart, V., Martin, P., Massoui, M., & Essassi, E. M. (2007). 1-benzyl-3-(2-(pyridin-2-yl)hydrazono)indolin-2-one. Molbank, 2007(2), M535. https://doi.org/10.3390/M535



