Identification of Select Fumonisin Forming Fusarium Species Using PCR Applications of the Polyketide Synthase Gene and its Relationship to Fumonisin Production in vitro
Abstract
:1. Introduction
2. Materials and Methods
Fungal Cultures
Design of gene-specific oligonucleotide primers
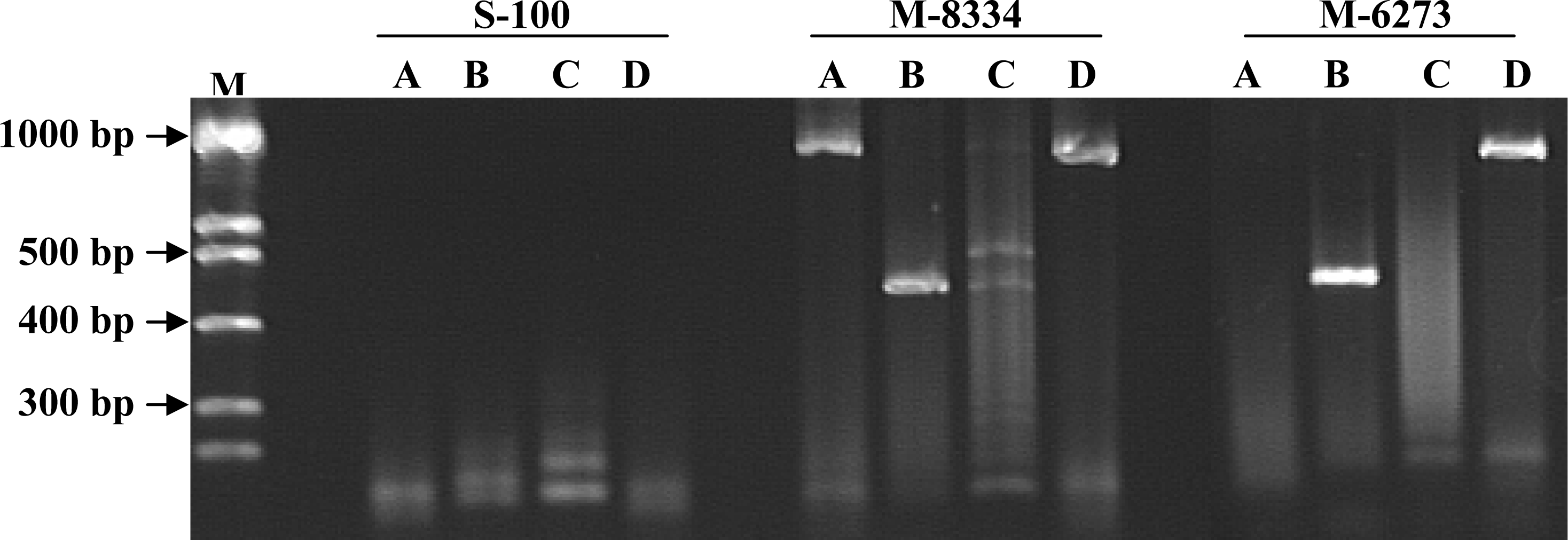
PCR Amplification
Fumonisin Production in vitro
Isolation of F. verticillioides from inoculated maize tissue
3. Results
4. Discussion
References and Notes
- White, DG. Compendium of corn diseases, 3rd ed; American Phytopathological Society: St. Paul, MN, 1999. [Google Scholar]
- Abbas, HK; Williams, WP; Windham, GL; Pringle, HC; Xie, W; Shier, WT. Aflatoxin and fumonisin contamination of commercial corn (Zea mays) hybrids in Mississippi. J Agric Food Chem 2002, 50, 5246–5254. [Google Scholar]
- Leslie, JF. Mating populations in Gibberella fujikuroi (Fusarium section Liseola). Phytopathology 1991, 81, 1058–1060. [Google Scholar]
- Britz, HT; Coutinho, A; Wingfield, MJ; Marasas, WFO; Gordon, TR; Leslie, JF. Fusarium subglutinans f. sp. pini represents a distinct mating population in the Gibberella fujikuroi species complex. Appl Environ Microbiol 1999, 65, 198–1201. [Google Scholar]
- Leslie, JF. Interfertility of two mating populations in the Gibberella fujikuroi species complex. Euro J Plant Pathol 2004, 110, 611–618. [Google Scholar]
- Leslie, JF. Gibberella fujikuroi: available populations and variable traits. Can J Botany 1995, 73(Suppl. 1), S282–S291. [Google Scholar]
- Munkvold, GP; Hellmich, RL; Showers, WB. Reduced Fusarium ear and symptomless infection in kernels of maize genetically engineered for European cornborer resistance. Phytopathology 1997, 87, 1071–1077. [Google Scholar]
- Foley, DC. Systematic infection of corn by Fusarium moniliforme. Phytopathology 1999, 68, 1331–1335. [Google Scholar]
- Bacon, CW; Hinton, DM; Richardson, MD. A corn seedling assay for resistance to Fusarium moniliforme. Plant Dis 1994, 78, 302–305. [Google Scholar]
- Bezuidonhout, SC; Gelderblom, WCA; Gorst-Allman, CP; Horak, RM; Marasas, WFO. Structure elucidation of fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Chem Commun 1988, 52, 743–745. [Google Scholar]
- Gelderblom, WCA; Jaskiewicz, K; Marasas, WFO; Thiel, PG; Horak, MJ; Vleggar, R; Kriek, NPK. Fumonisins - novel mycotoxins with cancer promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol 1988, 54, 1806–1811. [Google Scholar]
- Riley, TR; Norred, WP; Bacon, CW. Fungal toxins in food: recent concerns. Annu Rev Nutr 1993, 13, 167–189. [Google Scholar]
- Abbas, HKT; Tanaka, T; Shier, WT. Biological activities of synthetic analogs of Alternaria alternata AAL-toxin and fumonisin in plant and mammalian cell cultures. Phytochemistry 1995, 40, 1681–1689. [Google Scholar]
- Shier, WT; Abbas, HK; Badria, FA. Complete structure of the sphingosine analog mycotoxins fumonisin B1 and AAL-toxin TA:absolute configuration of the side chains. Tetrahedron Lett 1995, 36, 1571–1574. [Google Scholar]
- Proctor, RH; Desjardins, AE; Plattner, RD; Hohn, TM. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genet Biol 1999, 27, 100–112. [Google Scholar]
- Desjardins, AE; Munkvold, GP; Plattner, RD; Proctor, RH. Fum1: a gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliforme in field tests. Mol Plant Microbe In 2002, 15, 1157–1164. [Google Scholar]
- Proctor, RH; Brown, DW; Plattner, RD; Desjardins, AE. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet Biol 2003, 38, 237–249. [Google Scholar]
- CAST (Council for Agriculture Science and Technology), Mycotoxins risks in plant, animal, and human systems; CAST, Task Force Report 139: Ames, IA, 2003.
- NTP (National Toxicology Program), Toxicology and carcinogenesis studies on fumonisin B1 in F344/N rats and B6CF1 mice (feed studies); Technical Report Series, n.496. NIH Publication No. 99-3955; U.S. Department of Health and Human Services, National Institutes of Health: Research Triangle Park, NC, 1999.
- Nelson, PE; Desjardins, AE; Plattner, RD. Fumonisins, mycotoxins produced by Fusarium species: Biology, chemistry and significance. Annu Rev Phytopathol 1993, 31, 233–252. [Google Scholar]
- Thiel, PG; Marasas, WFO; Sydenham, EW; Shepard, GS; Gelderblom, WCA; Nieuwenhuis, JJ. Survey of fumonisin production by Fusarium species. Appl Environ Microbiol 1991, 57, 1089–1093. [Google Scholar]
- Rheeder, JP; Marasas, WFO; Vismer, HF. Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol 2002, 68, 2101–2105. [Google Scholar]
- Headrick, JM; Pataky, JK. Maternal influence on the resistance of sweet corn lines to kernel infection by Fusarium moniliforme. Phytopathology 1991, 81, 268–274. [Google Scholar]
- Booth, C. The genus Fusarium; Commonwealth Mycological Institute: Kew, United Kingdom, 1971. [Google Scholar]
- Grimm, C; Geisen, R. A PCR-ELISA for the detection of potential fumonisin producing Fusarium species. Lett Appl Microbiol 1988, 26, 456–462. [Google Scholar]
- Innis, MA; Gelfand, DH; Sninsky, JJ; White, TJ. PCR Protocols: A guide to method and applications; Innis, D, Gelfand, H, Sninsky, JJ, Eds.; Academic Press, Inc: N.Y., 1990. [Google Scholar]
- Bruns, TD; White, TJ; Taylor, JW. Fungal molecular systematics. Annu Rev Ecol and Syst 1991, 22, 525–564. [Google Scholar]
- Bateman, GL; Ward, E; Antoniwi, JF. Identification of Gaeumannomycos graminis var. tritici and G. graminis var. avenae using a DNA probe and non-molecular methods. Mycol Res 1992, 96, 737–742. [Google Scholar]
- Zhang, AW; Riccioni, GL; Chen, L; Ma, WD; Petersen, WL. Using PCR to distinguish Diaporthe phaseolorum and Phompsis longicolla from other soybean fungal pathogens and to detect them in soybean tissues. Plant Dis 1997, 81, 1143–1149. [Google Scholar]
- Beck, JJ; Ligon, JM. Polymerase chain reaction assays for the detection of Stagonospora nodorum and Septoria tritici in wheat. Phytopathology 1995, 85, 319–324. [Google Scholar]
- Schilling, AG; Moller, EM; Geiger, HH. Polymerase chain reaction-based assays for the detection for species-specific detection of Fusarium culmorum, F. graminearum, and F. avenaceum. Phytopathology 1996, 86, 515–522. [Google Scholar]
- Guthrie, PAI; Magill, GW; Fredericksen, RA; Oduody, GN. Random amplified polyorphic DNA markers: a system for identifying and differentiating isolates of Colletotrichum graminicola. Phytopathology 1992, 82, 832–835. [Google Scholar]
- Hornby, D; Bateman, G. Take-all of Cereals; Home-Grown Cereals Authority Research Review no. 2. Home-Grown Cereals Authority: London, 1991. [Google Scholar]
- Williams, JGK; Kubelik, AR; Rafalski, JA; Tingey, SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 1990, 18, 615–643. [Google Scholar]
- White, TJ; Bruns, T; Lee, S; Taylor, J. Amplification of direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A guide to method and applications. Innis, D, Gelfand, H, Sninsky, JJ, Eds.; Academic Press, Inc: New York, 1990. [Google Scholar]
- O’Donnell, K; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol and Phylogenet Evol 1997, 7, 103–116. [Google Scholar]
- O’Donnell, K; Cigelnik, E; Nirenberg, H. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar]
- Bayman, P; Cotty, PJ. Genetic diversity in Aspergillus flavus: association with aflatoxin production and morphology. Can J Botany 1993, 71, 23–34. [Google Scholar]
- Jones, MJ; Dunkle, LD. Analysis of Cochliobulus carbonum races by PCR amplification with arbitrary and gene-specific primers. Phytopathology 1993, 83, 366–370. [Google Scholar]
- González-Jaén, MT; Mirete, S; Patiňo, B; López-Errasquin, E; Vázquez, C. Genetic markers for the analysis of variability and for production of specific diagnotic sequences in fumonisin-producing strains of Fusarium verticillioides. Euro J of Plant Path 2004, 110, 525–532. [Google Scholar]
- Waalwijk, C; van der Heide, R; deVries, I; van der Lee, T; Schoen, C; Costrel-de Corainville, G; Häuser-Hahn, I; Kastelein, P; Köhl, J; Lonnet, P; Demarquet, T; Kema, GHJ. Quantitative detection of Fusarium species in wheat using TaqMan. Eur J Plant Pathol 2004, 110, 481–494. [Google Scholar]
- Bluhm, BM; Flaherty, JE; Cousin, MA; Woloshuk, CP. Multiplex polymerase chain reaction assay for the differential detection of trichothecene- and fumonisin-producing species of Fusarium in cornmeal. J Food Protect 2002, 65, 1955–1961. [Google Scholar]
- Bluhm, BM; Cousin, MA; Woloshuk, CP. Multiplex real-time PCR detection of fumonisin-producing and trichothecene-producing groups of Fusarium species. J Food Protect 2004, 67, 536–543. [Google Scholar]
- Patiňo, B; Mirete, S; González-Jaén, MT; Mulé, EG; Vázquez, C. PCR detection assay of fumonisin-producing Fusarium verticillioides stains. J. Food Protect 2004, 67, 1278–1283. [Google Scholar]
- Shim, WB; Woloshuk, CP. Nitrogen repression of fumonisin B1 biosynthesis in Gibberella fujikuroi. FEMS Microbiol Lett 1999, 177, 109–116. [Google Scholar]
- Abbas, HK; Cartwright, RD; Xie, W; Mirocha, CJ; Richard, JL; Dvorak, TJ; Sciumbato, GL; Shier, WT. Mycotoxin production by Fusarium proliferatum isolates from rice with Fusarium sheath rot disease. Mycopathologia 1999, 147, 97–104. [Google Scholar]
- Plattner, RD. HPLC/MS Analysis of Fusarium mycotoxins, fumonisins and deoxynivalenol. Nat Toxins 1999, 7, 365–370. [Google Scholar]
- Geiser, DM; Jiménez-Gasco, MD; Kang, S; Makalowska, I; Veeraraghavan, N; Ward, TJ; Zhang, N; Kuldau, GA; O’Donnell, K. Fusarium-ID v. 1.0: A DNA sequence database for identifying Fusarium. Europ J Plant Pathol 2004, 110, 473–479. [Google Scholar]
- Nelson, PE; Plattner, RD; Shackelford, DD; Desjardins, AE. Production of fumonisin by Fusarium moniliforme strains from various substrates and geographic areas. Appl Environ Micobiol 1991, 57, 2410–2412. [Google Scholar]
- Falasconi, M; Gobbi, E; Pardo, M; Dell Torre, M; Bresciani, A; Sberveglieri, G. Sensor Actuat. B-Chem. Detection of toxigenic strains of Fusarium verticillioides in corn by electronic olfactory system. 2005, 108, 250–257. [Google Scholar]
- O’Donnell, K. Ribosomal DNA internal transcribed spacers are highly divergent in the phytopathogenic ascomycete Fusarium sambusinum (Gibberella pulicaris). Curr Genet 1992, 22, 213–220. [Google Scholar]
- O’Donnell, K; Gray, LE. Phylogenetic relationships of the soybean sudden death syndrome pathogen Fusarium solani f. sp. solani inferred from rDNA sequence data and PCR primers for its identification. Mol Plant Microbe In 1995, 5, 709–716. [Google Scholar]
- Bezuidonhout, SC; Prinsloo, M; Vanderwalt, AM. Multiplex PCR-based detection of potential fusarium producing Fusarium in traditional African vegetables. Environ Toxicol 2006, 21, 360–366. [Google Scholar]
- Kerenyi, Z; Keller, K; Hornok, L; Leslie, JF. Molecular standardization of mating type terminology in the Gibberella fujkuroi species complex. Appl Environ Micobiol 1999, 65, 4071–4076. [Google Scholar]
- Desjardins, AE; Proctor, RH. In Fusarium: Biochemistry and Genetics of Fusarium Toxins; Paul E. Nelson Memorial Symposium; Summerall, BA, Leslie, JF, Backhouse, D, Bryden, WL, Burgess, LW, Eds.; The American Phytopathological Society: St. Paul, MN, 2001. [Google Scholar]
- Chulze, SN; Ramirez, ML; Torres, A; Leslie, JF. Genetic variation in Fusarium section Liseola from no-till maize in Argentina. Appl Environ Microbiol 2000, 66, 5312–5315. [Google Scholar]
- Desjardins, AE; Plattner, RD; Proctor, RH. Fumonisins in food: Genetic and biochemical aspects of fumonisin production; Jackson, LS, Ed.; Plenum Press: NY, 1996. [Google Scholar]
- Mule, G; Susca, A; Stea, G; Moretti, A. Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEMS Microbiol Lett 2004, 230, 235–240. [Google Scholar]
- Miller, SD. Factors that affect the occurrence of fumonisin. Environ Health Persp 2001, 109(Suppl.-2), 321–324. [Google Scholar]
| Set | Primer | Sequence | AF155773 position (bp) | Expected fragment size (bp) |
|---|---|---|---|---|
| A | Fum1F
Fum4R | GAGGCCCGAGCGAGCACTGG
CCAGCCGCGGAAATTAGGGATGTG | 24829–24849
26261–26284C | 1456 |
| B | Fum5F
Fum6R | GTCCTACGCGATACATCCCACCACAAT
GATCAAGCTCGGGGCCGTCGTTCATAG | 25750–25776
26142–26168C | 419 |
| C | Fum5F
Fum4R | GTCCTACGCGATACATCCCACCACAAT
CCAGCCGCGGAAATTAGGGATGTG | 25750–25776
26261–26284C | 534 |
| D | Fum1F
Fum6R | CGAGGCCCGAGCGAGCACTGG
GATCAAGCTCGGGGCCGTCGTTCATAG | 24829–24849
26142–26168C | 1340 |
| Isolate Number | Species/Host a | Location | PCR Primer Results b | Toxin Production c | ||
|---|---|---|---|---|---|---|
| B | D | Liquid Medium | Rice Medium | |||
| F1 (FCRB13) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | – | + |
| F7 (FCRB14) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | – | + |
| F13 (FCRB15) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | + | + |
| NRRL 20956 (FCRB7) | Fusarium verticillioides2/ unk | + | + | + | + | |
| NRRL 20960 (FCRB8) | Fusarium verticillioides2/ unk | + | + | + | + | |
| NRRL 20984 (FCRB9) | Fusarium verticillioides2/ unk | + | – | – | + | |
| NRRL 22001 (FCRB10) | Fusarium verticillioides2/ unk | + | + | + | + | |
| NRRL 22050 (FCRB11) | Fusarium verticillioides2/ unk | + | + | + | + | |
| NRRL 22052 (FCRB12) | Fusarium verticillioides2/ unk | + | + | – | + | |
| M-2326 (FCRB42) | Fusarium verticillioides (syn. F. moniliforme1)/maize | USA/Maryland | + | – | – | + |
| M-3441 (FCRB43) | Fusarium verticillioides 1/ soil debris | USA/Texas | – | + | – | – |
| M-5496 (FCRB44) | Fusarium verticillioides 1/ maize | NEPAL/Kathmondu | + | + | – | – |
| M-5697 (FCRB46) | Fusarium verticillioides 1/ maize | USA IA Ankeny | + | + | – | + |
| M-6092 (FCRB48) | Fusarium verticillioides 1 | USA/Iowa | + | + | – | + |
| M-6273 (FCRB49) | Fusarium verticillioides 1 | USA/Iowa | + | + | – | + |
| M-6562 (FCRB50) | Fusarium verticillioides 1/ maize | + | – | – | + | |
| M-8334 (FCRB52) | Fusarium verticillioides 1/ maize | + | + | – | + | |
| M-8335 (FCRB53) | Fusarium verticillioides 1/ maize | + | – | – | + | |
| MSF1 (FCRB34) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | – | + |
| MSF2 (FCRB35) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | + | + |
| MSF3 (FCRB36) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | + | + |
| MSF4 (FCRB37) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | + | + |
| MSF5 (FCRB38) | Fusarium verticillioides3 | USA/Mississippi | + | + | + | + |
| MSF6 (FCRB39) | Fusarium verticillioides3/ maize | USA/Mississippi | + | + | – | + |
| NRRL 25006 (FCRB1) | Fusarium proliferatum2/ unk | + | + | – | + | |
| NRRL 22025 (FCRB4) | Fusarium proliferatum2/ unk | – | – | – | – | |
| SF2-3 (FCRB28) | Fusarium proliferatum3/ seed | USA/Minnesota | – | – | – | – |
| SF4-3 (FCRB29) | Fusarium proliferatum3/ seed | USA/Mississippi | + | – | – | – |
| T10 (FCRB31) | Fusarium proliferatum3/ rice | USA/Texas | + | – | – | – |
| M-5608 (FCRB45) | Fusarium proliferatum1/ wheat | NEPAL Kathmandu | + | – | – | + |
| M-5978 (FCRB47) | Fusarium proliferatum1/ sorghum | Nigeria/Kaduna State | + | – | – | – |
| M-7444 (FCRB51) | Fusarium proliferatum1/ maize | NEPAL/Kathmandu | + | – | – | – |
| M-1977 (FCRB41) | Fusarium proliferatum1/ maize | USA/Pennsylvania | + | – | – | – |
| NRRL 22003 (FCRB5) | Fusarium proliferatum2/ maize | USA/Mississippi | + | – | – | + |
| NRRL 22032 (FCRB6) | Fusarium proliferatum2 / maize | USA/Mississippi | + | – | – | + |
| SF408 (FCRB22) | Fusarium proliferatum4/ soil | USA/Mississippi | + | – | – | + |
| SF 381 (FCRB21) | Fusarium equisiti4/ soil | USA/Minnesota | – | – | – | + |
| PF15 (FCRB17) | Fusarium oxysporum4/ root | USA/Mississippi | + | – | – | – |
| PF31 (FCRB16) | Fusarium oxysporum4/ root | USA/Minnesota | – | – | – | – |
| PF78 (FCRB18) | Fusarium oxysporum4/ soil | USA/Minnesota | – | – | – | – |
| PF41 (FCRB19) | Fusarium graminearum4/ root | USA/Minnesota | – | – | – | – |
| FPO15-3 (FCRB27) | Fusarium polyphialidicum4/ seed | USA/Minnesota | – | – | – | – |
| S 100 (FCRB54) | Fusarium solani4/ sorghum | USA/Texas | – | – | – | – |
| S 1091 (FCRB55) | Fusarium solani4/ soil | USA/Nebraska (NE) | – | – | – | + |
| CLRB-7 | Fusarium sambusinum/ cotton | USA/Mississippi | – | – | – | – |
| CLRB-8 | Fusarium sambusinum/ soybean | USA/Mississippi | – | – | – | + |
| CLRB-3 | Aspergillus flavus/ maize | USA/Mississippi | – | – | – | – |
| CLRB-5 | Curvularia lunata/ cotton | USA/Mississippi | – | – | – | – |
| CLRB-11 | Penicillium sp. / cotton | USA/Mississippi | – | – | – | – |
| CLRB-19 | Basidiomycete/ soybean | USA/Mississippi | – | – | – | – |
| CLRB-20 | Basidiomycete/ soybean | USA/Mississippi | – | – | – | – |
| CLRB-31 | Zygomycete (Absidia sp.) / soybean debris | USA/Mississippi | – | – | – | – |
| CLRB-38 | Neocosmospora vasinfecta/ soybean | USA/Mississippi | – | – | – | – |
| CLRB-40 | Phoma sp. / soybean | USA/Mississippi | – | – | – | – |
| CLRB-41 | Xylaria sp. / soybean debris | USA/Mississippi | – | – | – | – |
| CLRB-45 | Mucor hiemalis/ unk | USA/Mississippi | – | – | – | – |
| CLRB-49 | Verticillium sp. / cotton | USA/Mississippi | – | – | – | – |
| CLRB-50 | Rhizoctoria zeae/ soybean debris | USA/Mississippi | – | – | – | – |
| CLRB-54 | Pythium sp. / soybean debris | USA/Mississippi | – | – | – | – |
| CLRB-55 | Pythium sp. / soybean debris | USA/Mississippi | – | – | – | – |
Share and Cite
Baird, R.; Abbas, H.K.; Windham, G.; Williams, P.; Baird, S.; Ma, P.; Kelley, R.; Hawkins, L.; Scruggs, M. Identification of Select Fumonisin Forming Fusarium Species Using PCR Applications of the Polyketide Synthase Gene and its Relationship to Fumonisin Production in vitro. Int. J. Mol. Sci. 2008, 9, 554-570. https://doi.org/10.3390/ijms9040554
Baird R, Abbas HK, Windham G, Williams P, Baird S, Ma P, Kelley R, Hawkins L, Scruggs M. Identification of Select Fumonisin Forming Fusarium Species Using PCR Applications of the Polyketide Synthase Gene and its Relationship to Fumonisin Production in vitro. International Journal of Molecular Sciences. 2008; 9(4):554-570. https://doi.org/10.3390/ijms9040554
Chicago/Turabian StyleBaird, Richard, Hamed K. Abbas, Gary Windham, Paul Williams, Sonya Baird, Peter Ma, Rowena Kelley, Leigh Hawkins, and Mary Scruggs. 2008. "Identification of Select Fumonisin Forming Fusarium Species Using PCR Applications of the Polyketide Synthase Gene and its Relationship to Fumonisin Production in vitro" International Journal of Molecular Sciences 9, no. 4: 554-570. https://doi.org/10.3390/ijms9040554
APA StyleBaird, R., Abbas, H. K., Windham, G., Williams, P., Baird, S., Ma, P., Kelley, R., Hawkins, L., & Scruggs, M. (2008). Identification of Select Fumonisin Forming Fusarium Species Using PCR Applications of the Polyketide Synthase Gene and its Relationship to Fumonisin Production in vitro. International Journal of Molecular Sciences, 9(4), 554-570. https://doi.org/10.3390/ijms9040554




