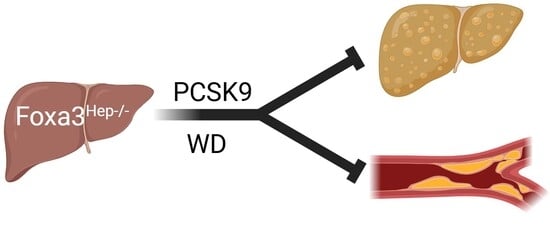
Loss of Hepatocyte FOXA3 Improves MASH and Atherosclerosis in Hyperlipidemic Ldlr-Deficient Mice
Abstract
1. Introduction
2. Results
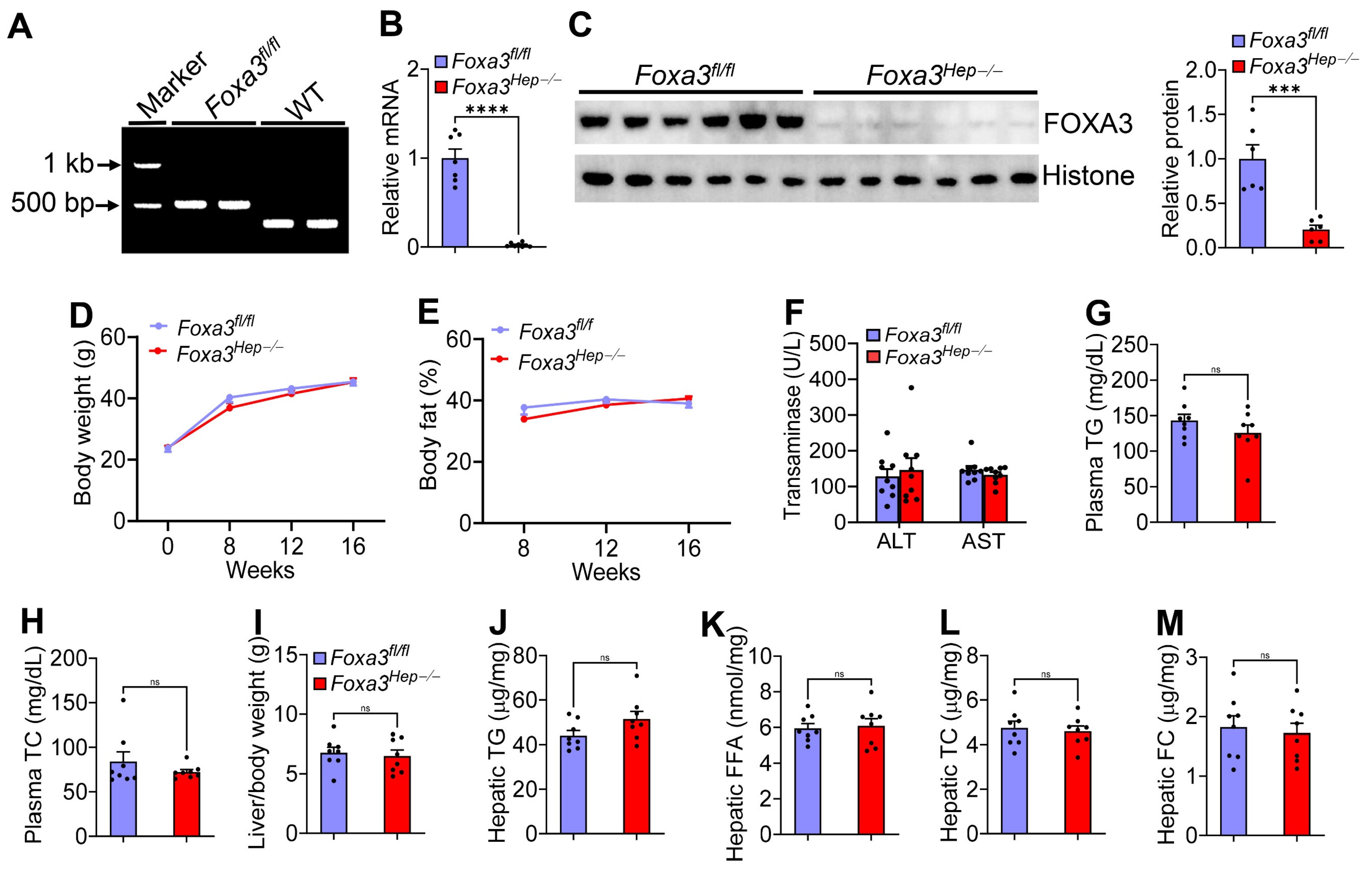
2.1. Genetic Loss of Hepatocyte Foxa3 Does Not Affect Hfcf Diet or Western Diet-Induced Masld
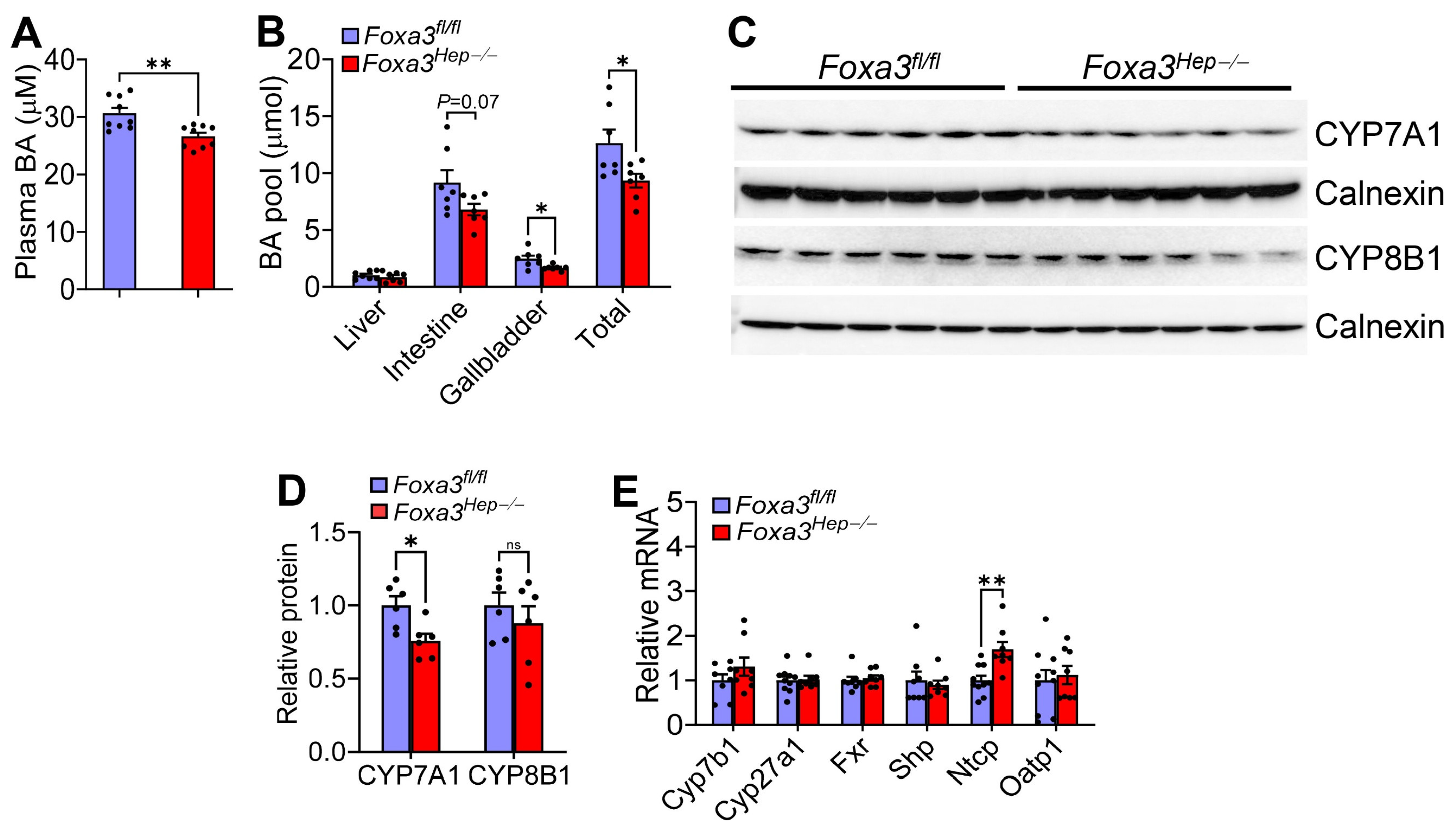
2.2. Loss of Hepatocyte Foxa3 Reduces the Bile Acid Pool Size in Hfcf Diet-Fed Mice
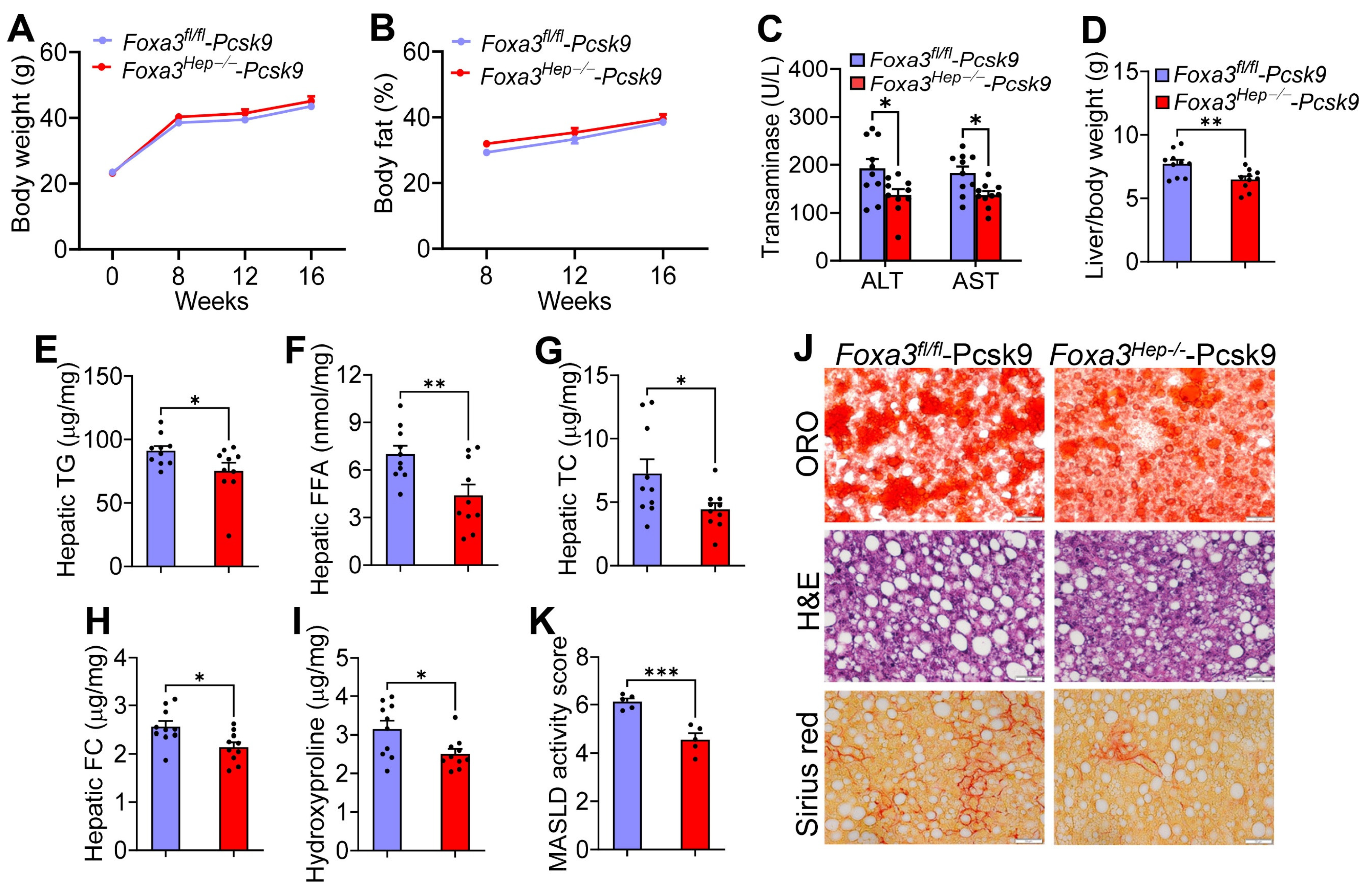
2.3. Genetic Ablation of Hepatocyte Foxa3 Improves Masld/Mash in Hyperlipidemic Ldlr-Deficient Mice
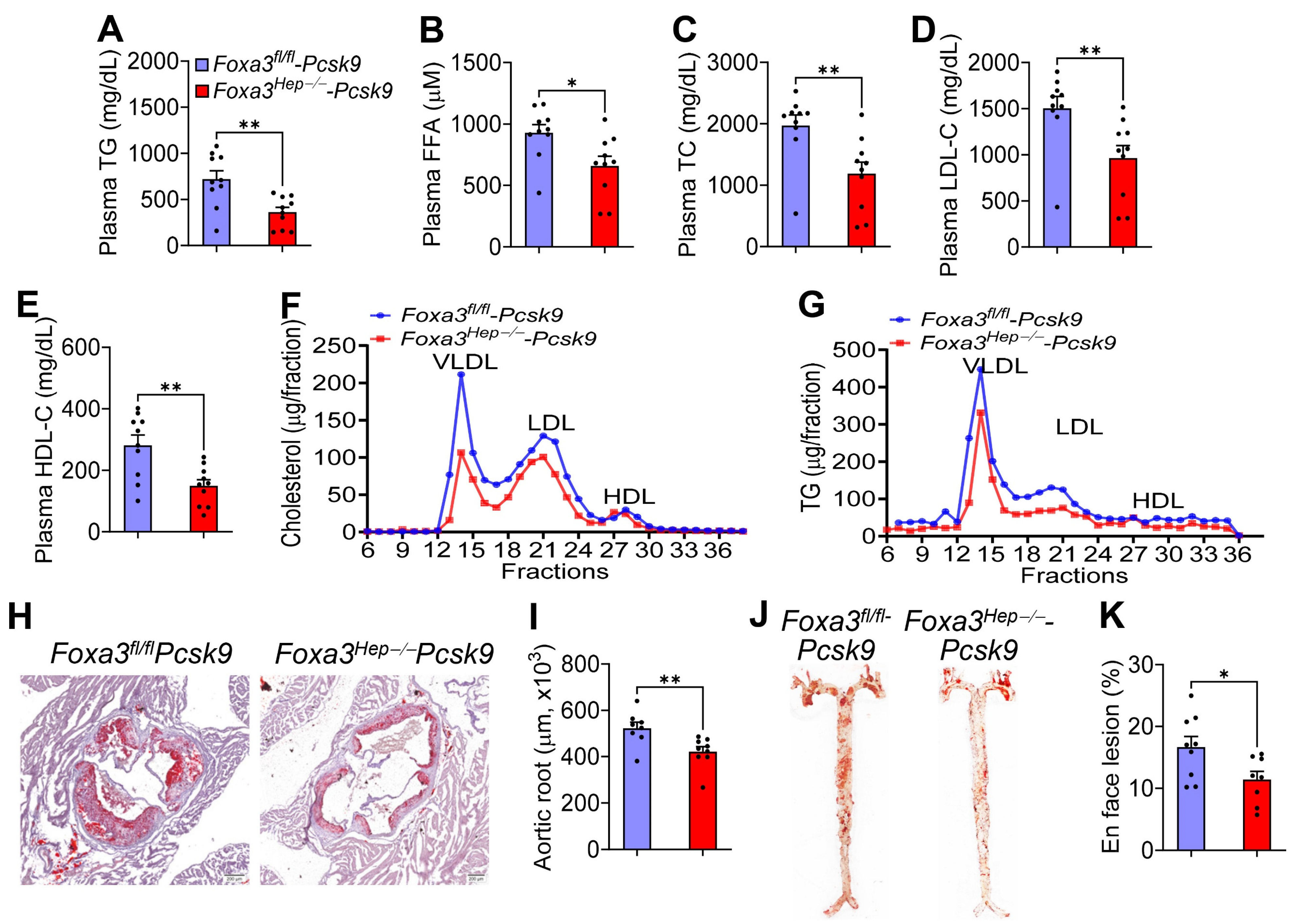
2.4. Loss of Hepatocyte Foxa3 Prevents the Development of Atherosclerosis in Hyperlipidemic Ldlr-Deficient Mice
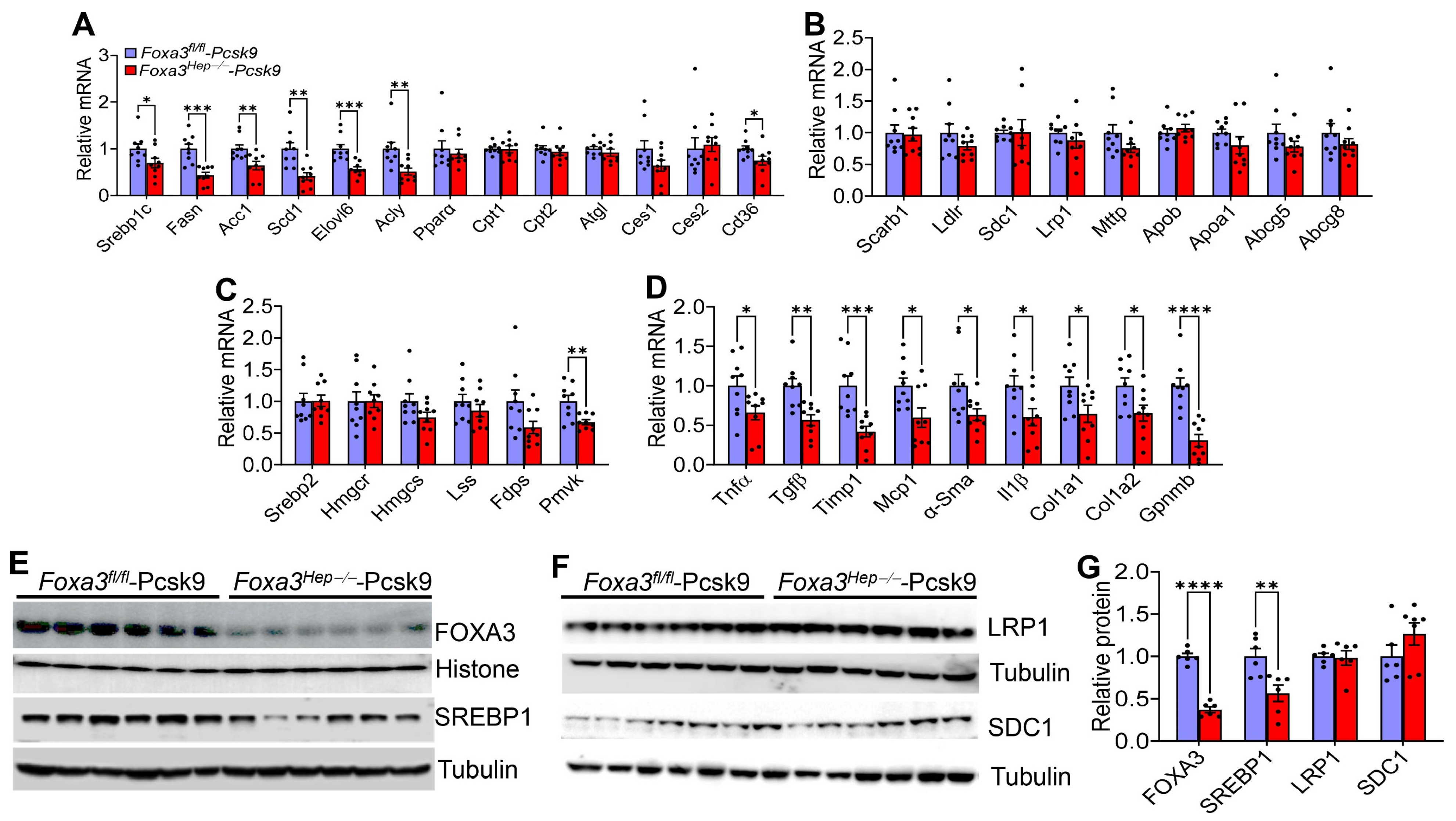
2.5. Loss of Hepatocyte Foxa3 Inhibits Lipogenic, Pro-Inflammatory, and Fibrogenic Genes in Hyperlipidemic Ldlr-Deficient Mice
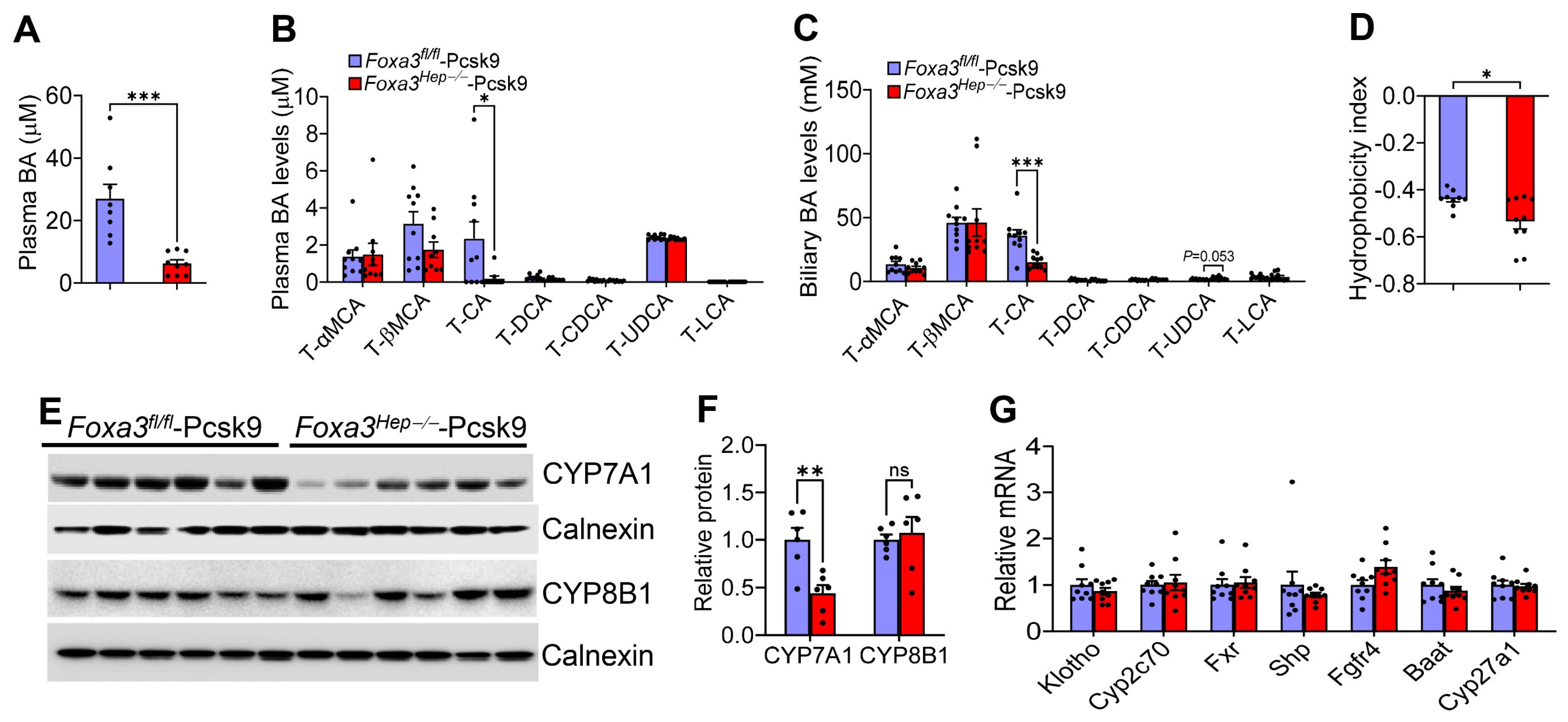
2.6. Loss of Hepatocyte Foxa3 Lowers Cholic Acid Levels and Reduces Bile Acid Hydrophobicity in Hyperlipidemic Ldlr-Deficient Mice
3. Discussion
4. Methods and Materials
4.1. Mice and Diets
4.2. Adeno-Associated Viruses (AAVs)
4.3. Quantitative Real-Time PCR
4.4. Western Blot Assays
4.5. Plasma and Liver Biochemistry
4.6. Bile Acid Levels, Bile Acid Pool Size, and Bile Acid Composition
4.7. Oil Red O (Oro), Hematoxylin and Eosin (H&e) Staining, and Picrosirius Red Staining
4.8. Body Composition and Energy Expenditure
4.9. Glucose Tolerance Test
4.10. Atherosclerotic Lesion Quantification
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in People With Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Ostergaard, L.H.; Long, M.T.; Kjaer, M.S.; Cali, A.M.G.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, B.; Meng, M.; Zhao, W.; Wang, D.; Yuan, Y.; Zheng, Y.; Qiu, J.; Li, Y.; Li, G.; et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Scearce, L.M.; Brestelli, J.E.; Sund, N.J.; Kaestner, K.H. Foxa3 (hepatocyte nuclear factor 3gamma) is required for the regulation of hepatic GLUT2 expression and the maintenance of glucose homeostasis during a prolonged fast. J. Biol. Chem. 2001, 276, 42812–42817. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Jadhav, K.; Zhu, Y.; Yin, L.; Zhang, Y. Hepatic Forkhead Box Protein A3 Regulates ApoA-I (Apolipoprotein A-I) Expression, Cholesterol Efflux, and Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Gopoju, R.; Wang, J.; Pan, X.; Hu, S.; Lin, L.; Clark, A.; Xu, Y.; Yin, L.; Wang, X.; Zhang, Y. Hepatic FOXA3 overexpression prevents Western diet-induced obesity and MASH through TGR5. J. Lipid Res. 2024, 65, 100527. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, L.; Guo, M.; Wang, D.; Meng, M.; Zhong, Y.; Zhang, Z.; Lin, Y.; Liu, C.; Wang, J.; et al. Characterisation of forkhead box protein A3 as a key transcription factor for hepatocyte regeneration. JHEP Rep. 2023, 5, 100906. [Google Scholar] [CrossRef]
- Dong, R.; Yang, Y.; Shen, Z.; Zheng, C.; Jin, Z.; Huang, Y.; Zhang, Z.; Zheng, S.; Chen, G. Forkhead box A3 attenuated the progression of fibrosis in a rat model of biliary atresia. Cell Death Dis. 2017, 8, e2719. [Google Scholar] [CrossRef]
- Nakamura, T.; Akiyoshi, H.; Shiota, G.; Isono, M.; Nakamura, K.; Moriyama, M.; Sato, K. Hepatoprotective action of adenovirus-transferred HNF-3gamma gene in acute liver injury caused by CCl(4). FEBS Lett. 1999, 459, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, K.H.; Hiemisch, H.; Schutz, G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol. Cell Biol. 1998, 18, 4245–4251. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Jadhav, K.; Pan, X.; Zhu, Y.; Hu, S.; Chen, S.; Chen, L.; Tang, Y.; Wang, H.H.; et al. Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism. Nat. Metab. 2021, 3, 59–74. [Google Scholar] [CrossRef]
- Bjorklund, M.M.; Hollensen, A.K.; Hagensen, M.K.; Dagnaes-Hansen, F.; Christoffersen, C.; Mikkelsen, J.G.; Bentzon, J.F. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ. Res. 2014, 114, 1684–1689. [Google Scholar] [CrossRef]
- Bertaggia, E.; Jensen, K.K.; Castro-Perez, J.; Xu, Y.; Di Paolo, G.; Chan, R.B.; Wang, L.; Haeusler, R.A. Cyp8b1 ablation prevents western diet-induced weight gain and hepatic steatosis due to impaired fat absorption. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E121–E133. [Google Scholar] [CrossRef] [PubMed]
- Chevre, R.; Trigueros-Motos, L.; Castano, D.; Chua, T.; Corliano, M.; Patankar, J.V.; Sng, L.; Sim, L.; Juin, T.L.; Carissimo, G.; et al. Therapeutic modulation of the bile acid pool by Cyp8b1 knockdown protects against nonalcoholic fatty liver disease in mice. FASEB J. 2018, 32, 3792–3802. [Google Scholar] [CrossRef]
- Slatis, K.; Gafvels, M.; Kannisto, K.; Ovchinnikova, O.; Paulsson-Berne, G.; Parini, P.; Jiang, Z.Y.; Eggertsen, G. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J. Lipid Res. 2010, 51, 3289–3298. [Google Scholar] [CrossRef]
- Li, R.; Palmiotti, A.; de Vries, H.D.; Hovingh, M.V.; Koehorst, M.; Mulder, N.L.; Zhang, Y.; Kats, K.; Bloks, V.W.; Fu, J.; et al. Low production of 12alpha-hydroxylated bile acids prevents hepatic steatosis in Cyp2c70(−/−) mice by reducing fat absorption. J. Lipid Res. 2021, 62, 100134. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Chen, W.D.; Xu, Y.; Yin, L.; Ge, X.; Jadhav, K.; Adorini, L.; Zhang, Y. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor-controlled lipid homeostasis. Hepatology 2014, 59, 1761–1771. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.K.; Yin, L.; Zhang, Y. FXR Activation Increases Reverse Cholesterol Transport by Modulating Bile Acid Composition and Cholesterol Absorption. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef]
- Heuman, D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res. 1989, 30, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, X.; Heemstra, L.A.; Chen, W.D.; Xu, J.; Smith, J.L.; Ma, H.; Kasim, N.; Edwards, P.A.; Novak, C.M. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol. Endocrinol. 2012, 26, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cassim Bawa, F.N.; Zhu, Y.; Pan, X.; Wang, H.; Gopoju, R.; Xu, Y.; Zhang, Y. Loss of adipose ATF3 promotes adipose tissue lipolysis and the development of MASH. Commun. Biol. 2024, 7, 1300. [Google Scholar] [CrossRef] [PubMed]
- Mina, A.I.; LeClair, R.A.; LeClair, K.B.; Cohen, D.E.; Lantier, L.; Banks, A.S. CalR: A Web-Based Analysis Tool for Indirect Calorimetry Experiments. Cell Metab. 2018, 28, 656–666 e1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, H.; Hu, S.; Wang, J.; Gopoju, R.; Lin, L.; Gunawardana, L.; Wang, X.; Yin, L.; Zhang, Y. Loss of Hepatocyte FOXA3 Improves MASH and Atherosclerosis in Hyperlipidemic Ldlr-Deficient Mice. Int. J. Mol. Sci. 2026, 27, 1468. https://doi.org/10.3390/ijms27031468
Wang H, Hu S, Wang J, Gopoju R, Lin L, Gunawardana L, Wang X, Yin L, Zhang Y. Loss of Hepatocyte FOXA3 Improves MASH and Atherosclerosis in Hyperlipidemic Ldlr-Deficient Mice. International Journal of Molecular Sciences. 2026; 27(3):1468. https://doi.org/10.3390/ijms27031468
Chicago/Turabian StyleWang, Hui, Shuwei Hu, Jiayou Wang, Raja Gopoju, Li Lin, Lakshitha Gunawardana, Xinwen Wang, Liya Yin, and Yanqiao Zhang. 2026. "Loss of Hepatocyte FOXA3 Improves MASH and Atherosclerosis in Hyperlipidemic Ldlr-Deficient Mice" International Journal of Molecular Sciences 27, no. 3: 1468. https://doi.org/10.3390/ijms27031468
APA StyleWang, H., Hu, S., Wang, J., Gopoju, R., Lin, L., Gunawardana, L., Wang, X., Yin, L., & Zhang, Y. (2026). Loss of Hepatocyte FOXA3 Improves MASH and Atherosclerosis in Hyperlipidemic Ldlr-Deficient Mice. International Journal of Molecular Sciences, 27(3), 1468. https://doi.org/10.3390/ijms27031468