Recent Advances in Polyoxometalates Targeting Proteins Associated with Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Applications
Abstract
1. Introduction
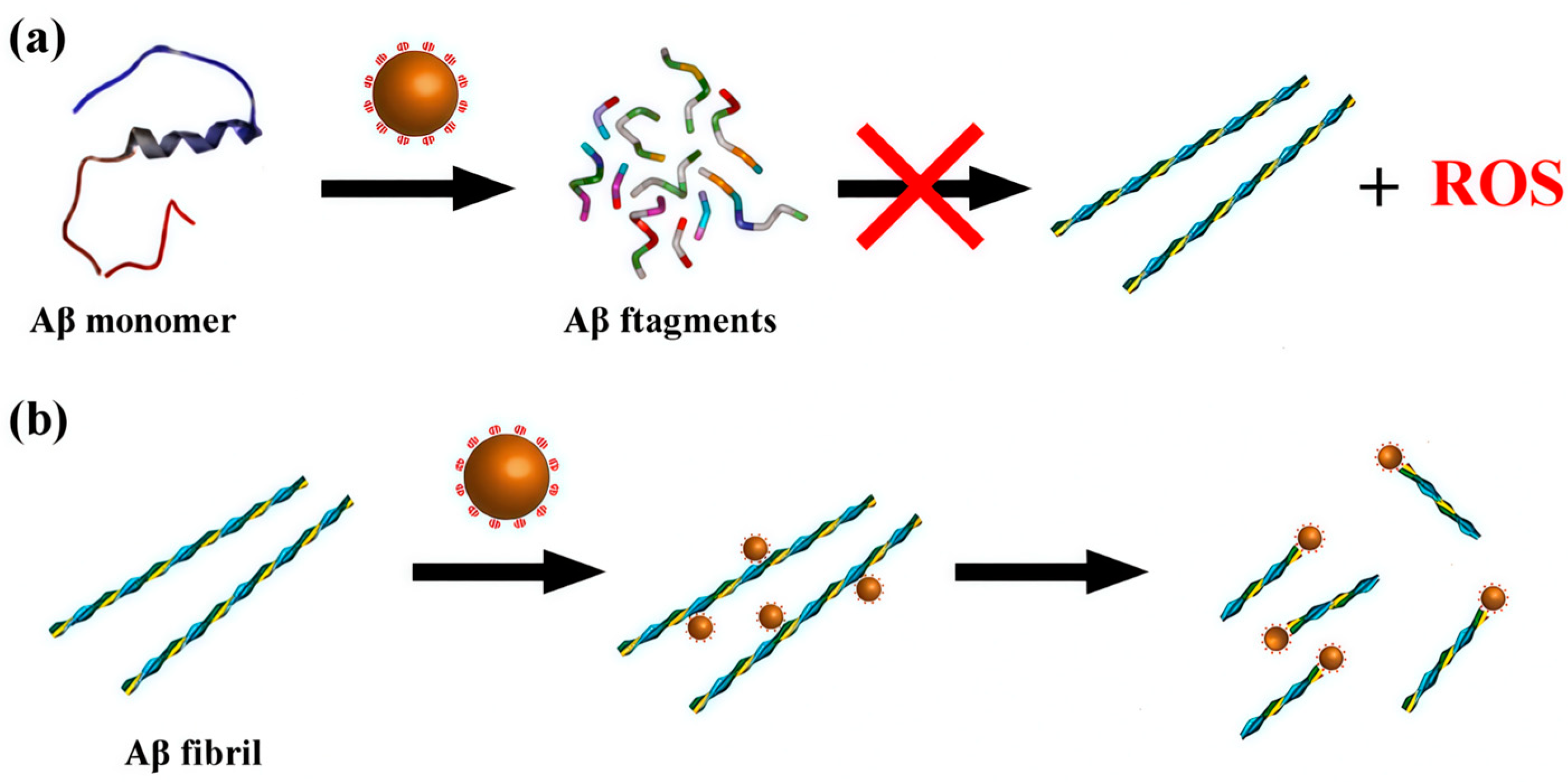
2. POMs in the Inhibition of Aβ Aggregation
2.1. Inhibition of Aβ Aggregation by Pure POMs
2.2. Transition Metal Substituted POMs
2.3. Inorganic-Organic Hybrid POMs
2.4. POM-Based Nanocomposites
3. Application of POMs in Phototherapy
3.1. Application of POMs in Photodynamic Therapy (PDT)
3.2. Application of POMs in Photothermal Therapy (PTT)
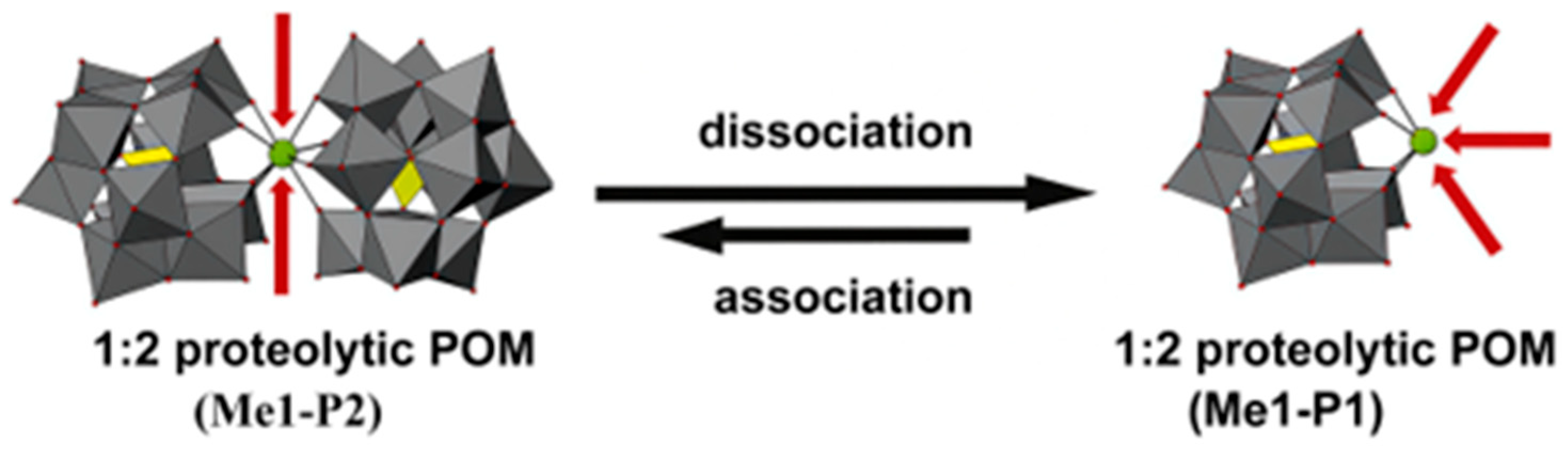
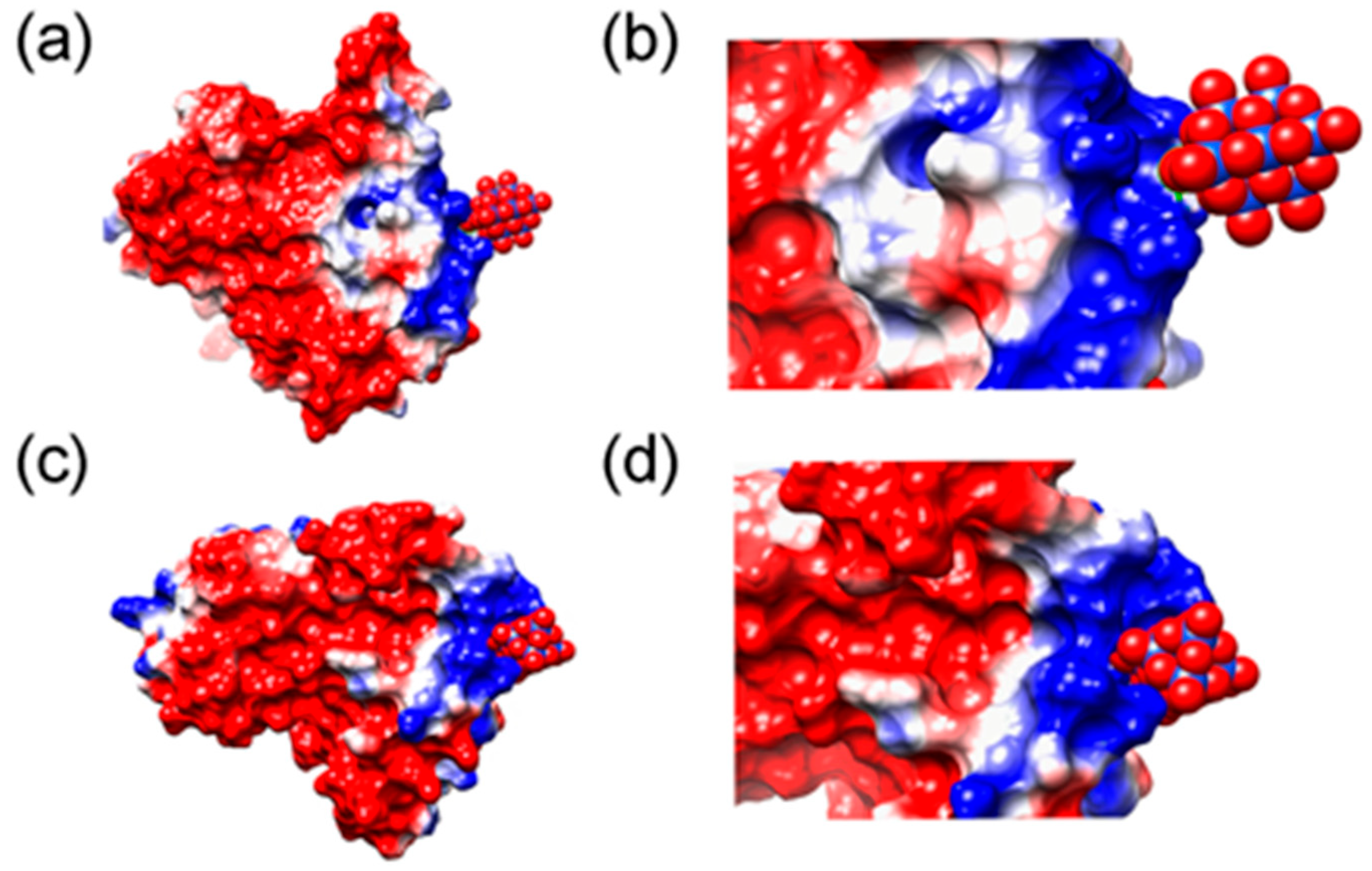
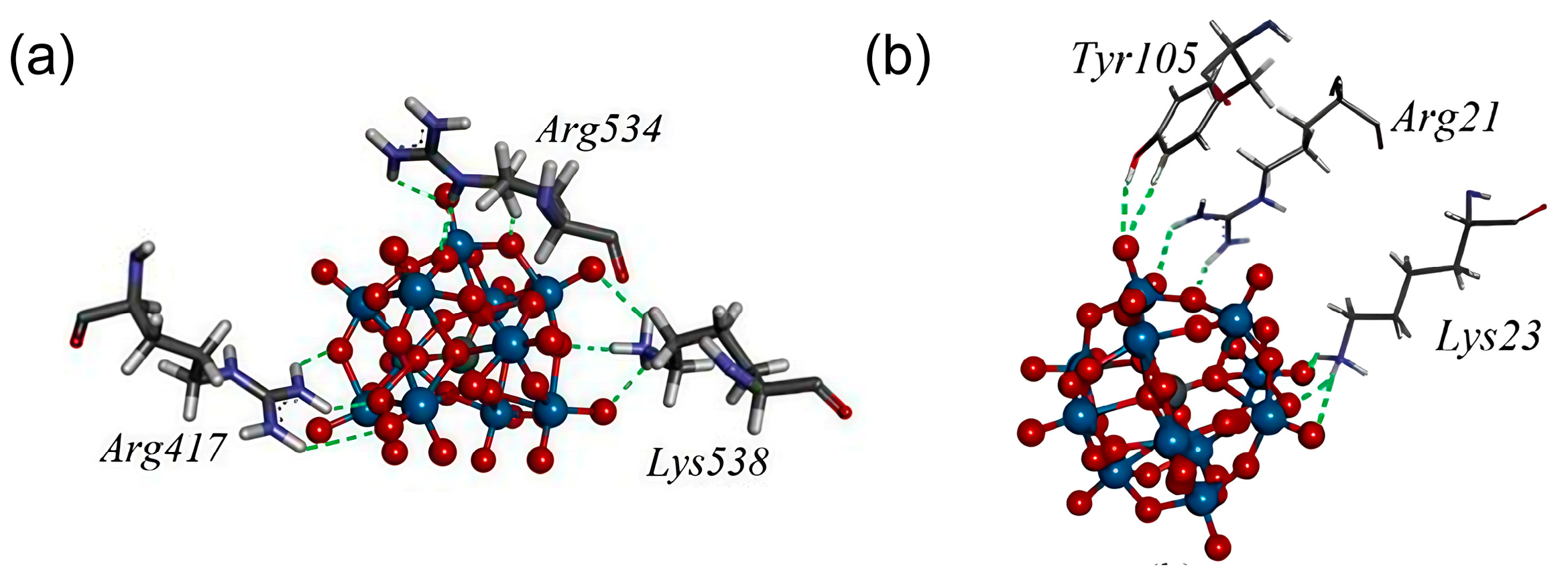
4. Application of POMs in Artificial Proteases
5. Progress and Application of POMs to Other Target Proteins in Alzheimer’s Disease Treatment
5.1. Targeting S100A9
5.2. Targeted Cholinesterase (CHE)
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef]
- Ben Zaken, K.; Bouhnik, R.; Omer, N.; Bloch, N.; Samson, A.O. Polyoxometalates bind multiple targets involved in Alzheimer’s disease. J. Biol. Inorg. Chem. 2025, 30, 299–309. [Google Scholar] [CrossRef]
- Umar, T.; Hoda, N. Alzheimer’s Disease: A Systemic Review of Substantial Therapeutic Targets and the Leading Multi-functional Molecules. Curr. Top. Med. Chem. 2018, 17, 3370–3389. [Google Scholar] [CrossRef]
- Fassbender, K.; Masters, C.; Beyreuther, K. Alzheimer’s disease: Molecular concepts and therapeutic targets. Naturwissenschaften 2001, 88, 261–267. [Google Scholar] [PubMed]
- Alam, J.; Sharma, L. Potential Enzymatic Targets in Alzheimer’s: A Comprehensive Review. Curr. Drug Targets 2019, 20, 316–339. [Google Scholar] [CrossRef]
- Abdallah, A.E. Review on anti-alzheimer drug development: Approaches, challenges and perspectives. RSC Adv. 2024, 14, 11057–11088. [Google Scholar] [CrossRef]
- Mal, S.S.; Banerjee, A.; Kortz, U. The cyclic 48-tungsto-8-phosphate [H7P8W48O184]33− Contant–Tézé polyanion and its derivatives [H6P4W24O94]18− and [H2P2W12O48]12−: Structural aspects and reactivity. Dalton Trans. 2025, 54, 5208–5233. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.J.; Parac-Vogt, T.N.; Quiñonero, D.; Prabhakar, R. Investigating Polyoxometalate–Protein Interactions at Chemically Distinct Binding Sites. J. Phys. Chem. B 2018, 122, 7219–7232. [Google Scholar] [CrossRef] [PubMed]
- Rambaran, M.A.; Gorzsás, A.; Holmboe, M.; Ohlin, C.A. Polyoxoniobates as molecular building blocks in thin films. Dalton Trans. 2021, 50, 16030–16038. [Google Scholar] [CrossRef]
- Tonkushina, M.O.; Belozerova, K.A.; Gagarin, I.D.; Adamova, L.V.; Terziyan, T.V.; Russkikh, O.V.; Ostroushko, A.A. Thermodynamics of the interaction between Keplerate-type polyoxometalate {Mo72Fe30} and vitamin B1. Thermochim. Acta 2022, 711, 179201. [Google Scholar] [CrossRef]
- Sap, A.; De Zitter, E.; Van Meervelt, L.; Parac-Vogt, T.N. Structural Characterization of the Complex between Hen Egg-White Lysozyme and ZrIV -Substituted Keggin Polyoxometalate as Artificial Protease. Chem. Eur. J. 2015, 21, 11692–11695. [Google Scholar] [CrossRef] [PubMed]
- Solé-Daura, A.; Goovaerts, V.; Stroobants, K.; Absillis, G.; Jiménez-Lozano, P.; Poblet, J.M.; Hirst, J.D.; Parac-Vogt, T.N.; Carbó, J.J. Probing Polyoxometalate—Protein Interactions Using Molecular Dynamics Simulations. Chem. Eur. J. 2016, 22, 15280–15289. [Google Scholar] [CrossRef]
- Vandebroek, L.; Mampaey, Y.; Antonyuk, S.; Van Meervelt, L.; Parac-Vogt, T.N. Noncovalent Complexes Formed Between Metal-Substituted Polyoxometalates and Hen Egg White Lysozyme. Eur. J. Inorg. Chem. 2019, 2019, 506–511. [Google Scholar] [CrossRef]
- Zhang, G.; Keita, B.; Craescu, C.T.; Miron, S.; De Oliveira, P.; Nadjo, L. Molecular Interactions between Wells−Dawson Type Polyoxometalates and Human Serum Albumin. Biomacromolecules 2008, 9, 812–817. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Duan, T.; Xu, C.; Qu, X. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer’s disease. Nat. Commun. 2014, 5, 3422. [Google Scholar] [CrossRef] [PubMed]
- Vandebroek, L.; Van Meervelt, L.; Parac-Vogt, T.N. Direct observation of the ZrIV interaction with the carboxamide bond in a noncovalent complex between Hen Egg White Lysozyme and a Zr-substituted Keggin polyoxometalate. Acta Crystallogr. C Struct. Chem. 2018, 74, 1348–1354. [Google Scholar] [CrossRef]
- Breibeck, J.; Bijelic, A.; Rompel, A. Transition metal-substituted Keggin polyoxotungstates enabling covalent attachment to proteinase K upon co-crystallization. Chem. Commun. 2019, 55, 11519–11522. [Google Scholar] [CrossRef]
- Blazevic, A.; Al-Sayed, E.; Roller, A.; Giester, G.; Rompel, A. Tris-Functionalized Hybrid Anderson Polyoxometalates: Synthesis, Characterization, Hydrolytic Stability and Inversion of Protein Surface Charge. Chem. Eur. J. 2015, 21, 4762–4771. [Google Scholar] [CrossRef]
- Zamolo, V.A.; Modugno, G.; Lubian, E.; Cazzolaro, A.; Mancin, F.; Giotta, L.; Mastrogiacomo, D.; Valli, L.; Saccani, A.; Krol, S. Selective Targeting of Proteins by Hybrid Polyoxometalates: Interaction Between a Bis-Biotinylated Hybrid Conjugate and Avidin. Front. Chem. 2018, 6, 278. [Google Scholar] [CrossRef] [PubMed]
- Arefian, M.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. A survey of the different roles of polyoxometalates in their interaction with amino acids, peptides and proteins. Dalton Trans. 2017, 46, 6812–6829. [Google Scholar] [CrossRef]
- Lentink, S.; Salazar Marcano, D.E.; Moussawi, M.A.; Vandebroek, L.; Van Meervelt, L.; Parac-Vogt, T.N. Fine-tuning non-covalent interactions between hybrid metal-oxo clusters and proteins. Faraday Discuss. 2023, 244, 21–38. [Google Scholar] [CrossRef]
- Vandebroek, L.; De Zitter, E.; Ly, H.G.T.; Conić, D.; Mihaylov, T.; Sap, A.; Proost, P.; Pierloot, K.; Van Meervelt, L.; Parac-Vogt, T.N. Protein-Assisted Formation and Stabilization of Catalytically Active Polyoxometalate Species. Chem. Eur. J. 2018, 24, 10099–10108. [Google Scholar] [CrossRef]
- Solé-Daura, A.; Poblet, J.M.; Carbó, J.J. Structure–Activity Relationships for the Affinity of Chaotropic Polyoxometalate Anions towards Proteins. Chem. Eur. J. 2020, 26, 5799–5809. [Google Scholar] [CrossRef]
- Breibeck, J.; Gumerova, N.I.; Boesen, B.B.; Galanski, M.S.; Rompel, A. Keggin-type polyoxotungstates as mushroom tyrosinase inhibitors-A speciation study. Sci. Rep. 2019, 9, 5183. [Google Scholar] [CrossRef] [PubMed]
- Vandebroek, L.; Noguchi, H.; Kamata, K.; Tame, J.R.H.; Van Meervelt, L.; Parac-Vogt, T.N.; Voet, A.R.D. Shape and Size Complementarity-Induced Formation of Supramolecular Protein Assemblies with Metal-Oxo Clusters. Cryst. Growth Des. 2021, 21, 1307–1313. [Google Scholar] [CrossRef]
- Greijer, B.H.; Nestor, G.; Eriksson, J.E.; Seisenbaeva, G.A.; Kessler, V.G. Factors influencing stoichiometry and stability of polyoxometalate—Peptide complexes. Dalton Trans. 2022, 51, 9511–9521. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Blazevic, A.; Roller, A.; Rompel, A. The Synthesis and Characterization of Aromatic Hybrid Anderson–Evans POMs and their Serum Albumin Interactions: The Shift from Polar to Hydrophobic Interactions. Chem. Eur. J. 2015, 21, 17800–17807. [Google Scholar] [CrossRef]
- Zhang, G.; Keita, B.; Craescu, C.T.; Miron, S.; De Oliveira, P.; Nadjo, L. Polyoxometalate Binding to Human Serum Albumin: A Thermodynamic and Spectroscopic Approach. J. Phys. Chem. B 2007, 111, 11253–11259. [Google Scholar] [CrossRef] [PubMed]
- Stroobants, K.; Saadallah, D.; Bruylants, G.; Parac-Vogt, T.N. Thermodynamic study of the interaction between hen egg white lysozyme and Ce(IV)-Keggin polyoxotungstate as artificial protease. Phys. Chem. Chem. Phys. 2014, 16, 21778–21787. [Google Scholar] [CrossRef]
- Zhang, G.; Keita, B.; Brochon, J.C.; De Oliveira, P.; Nadjo, L.; Craescu, C.T.; Miron, S. Molecular Interaction and Energy Transfer between Human Serum Albumin and Polyoxometalates. J. Phys. Chem. B 2007, 111, 1809–1814. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef]
- Geng, J.; Li, M.; Ren, J.; Wang, E.; Qu, X. Polyoxometalates as Inhibitors of the Aggregation of Amyloid β Peptides Associated with Alzheimer’s Disease. Angew. Chem. Int. Ed. 2011, 50, 4184–4188. [Google Scholar] [CrossRef] [PubMed]
- Konkova, A.V.; Savina, I.V.; Evtushok, D.V.; Pozmogova, T.N.; Solomatina, M.V.; Nokhova, A.R.; Alekseev, A.Y.; Kuratieva, N.V.; Eltsov, I.V.; Yanshole, V.V. Water-Soluble Polyoxometal Clusters of Molybdenum (V) with Pyrazole and Triazole: Synthesis and Study of Cytotoxicity and Antiviral Activity. Molecules 2023, 28, 8079. [Google Scholar] [CrossRef] [PubMed]
- Anyushin, A.V.; Kondinski, A.; Parac-Vogt, T.N. Hybrid polyoxometalates as post-functionalization platforms: From fundamentals to emerging applications. Chem. Soc. Rev. 2020, 49, 382–432. [Google Scholar] [CrossRef]
- Gao, N.; Du, Z.; Guan, Y.; Dong, K.; Ren, J.; Qu, X. Chirality-Selected Chemical Modulation of Amyloid Aggregation. J. Am. Chem. Soc. 2019, 141, 6915–6921. [Google Scholar] [CrossRef]
- Hu, S.; Ning, X.; Lv, J.; Wei, Y.; Zhang, H.; Li, M. Enantioselective modulation of amyloid burden and memory deficits by chiral polyoxometalates for Alzheimer’s disease treatment. Inorg. Chem. Front. 2023, 10, 5347–5356. [Google Scholar] [CrossRef]
- Hein, Z.M.; Karikalan, B.; Gopalakrishna, P.K.; Dhevi, K.; Alkatiri, A.; Hussan, F.; Mohd Moklas, M.A.; Jagadeesan, S.; Che Ramli, M.D.; Che Mohd Nassir, C.M.N. Toward a Unified Framework in Molecular Neurobiology of Alzheimer’s Disease: Revisiting the Pathophysiological Hypotheses. Mol. Neurobiol. 2026, 63, 282. [Google Scholar] [CrossRef] [PubMed]
- Perxés Perich, M.; Palma-Florez, S.; Solé, C.; Goberna-Ferrón, S.; Samitier, J.; Gómez-Romero, P.; Mir, M.; Lagunas, A. Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model. Nanomaterials 2023, 13, 2697. [Google Scholar] [CrossRef]
- Das, S. Alzheimer’s disease basics: We all should know. Neurol. Res. 2025, 1–30. [Google Scholar] [CrossRef]
- Wang, C.; Klechikov, A.G.; Gharibyan, A.L.; Wärmländer, S.K.T.S.; Jarvet, J.; Zhao, L.; Jia, X.; Shankar, S.K.; Olofsson, A.; Brännström, T. The role of pro-inflammatory S100A9 in Alzheimer’s disease amyloid-neuroinflammatory cascade. Acta Neuropathol. 2014, 127, 507–522, Correction in Acta Neuropathol. 2014, 128, 461. [Google Scholar] [CrossRef]
- Iqbal, J.; Barsukova-Stuckart, M.; Ibrahim, M.; Ali, S.U.; Khan, A.A.; Kortz, U. Polyoxometalates as potent inhibitors for acetyl and butyrylcholinesterases and as potential drugs for the treatment of Alzheimer’s disease. Med. Chem. Res. 2013, 22, 1224–1228. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.; Zhao, H.; Zhang, H.; Ren, J.; Qu, X. Polyoxometalates: Metallodrug agents for combating amyloid aggregation. Natl. Sci. Rev. 2024, 11, nwae226. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, L.; Han, F.; Zhang, G.; Ma, Y.; Yao, J.; Keita, B.; De Oliveira, P.; Nadjo, L. Inhibition of amyloid-β protein fibrillization upon interaction with polyoxometalates nanoclusters. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 97–101. [Google Scholar] [CrossRef]
- Qu, X.; Xu, K.; Zhao, C.; Song, X.; Li, J.; Li, L.; Nie, W.; Bao, H.; Wang, J.; Niu, F. Genotoxicity and acute and subchronic toxicity studies of a bioactive polyoxometalate in Wistar rats. BMC Pharmacol. Toxicol. 2017, 18, 26. [Google Scholar] [CrossRef]
- Lentink, S.; Salazar Marcano, D.E.; Moussawi, M.A.; Parac-Vogt, T.N. Exploiting Interactions between Polyoxometalates and Proteins for Applications in (Bio)chemistry and Medicine. Angew. Chem. Int. Ed. 2023, 135, e202303817. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.; Ren, J.; Wang, E.; Qu, X. Photodegradation of β-sheet amyloid fibrils associated with Alzheimer’s disease by using polyoxometalates as photocatalysts. Chem. Commun. 2013, 49, 11394. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, L.; Zheng, C.; Zheng, W.; Zhang, J.; Zhou, Y.; Liu, J. Mo polyoxometalate nanoclusters capable of inhibiting the aggregation of Aβ-peptide associated with Alzheimer’s disease. Nanoscale 2014, 6, 6886–6897. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Peng, R.; Xia, L.; Zhuang, G. Active Learning-Assisted Exploration of [PO40Mo12]3− for Alzheimer’s Therapy Insights. Adv. Sci. 2025, 12, e08702. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hua, J.; Zheng, P.; Tian, Y.; Kang, S.; Chen, J.; Duan, Y.; Ma, X. A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with β-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding. Inorganics 2023, 11, 442. [Google Scholar] [CrossRef]
- Ma, X.; Hua, J.; Wang, K.; Zhang, H.; Zhang, C.; He, Y.; Guo, Z.; Wang, X. Modulating Conformation of Aβ-Peptide: An Effective Way to Prevent Protein-Misfolding Disease. Inorg. Chem. 2018, 57, 13533–13543. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Wan, K.; Sun, T.; Zheng, N.; Zhu, F.; Ma, J.; Jiao, J.; Li, T.; Ni, J. Organoplatinum-Substituted Polyoxometalate Inhibits β-amyloid Aggregation for Alzheimer’s Therapy. Angew. Chem. Int. Ed. 2019, 58, 18032–18039. [Google Scholar] [CrossRef]
- Hua, J.; Wei, X.; Ma, X.; Jiao, J.; Chai, B.; Wu, C.; Zhang, C.; Niu, Y. A {Cd4Cl2O14 } cluster functionalized sandwich-type tungstoarsenate as a conformation modulator for misfolding Aβ peptides. CrystEngComm 2022, 24, 1171–1176. [Google Scholar] [CrossRef]
- Gao, N.; Liu, Z.; Zhang, H.; Liu, C.; Yu, D.; Ren, J.; Qu, X. Site-Directed Chemical Modification of Amyloid by Polyoxometalates for Inhibition of Protein Misfolding and Aggregation. Angew. Chem. Int. Ed. 2022, 61, e202115336. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Peng, K.; Han, L.; Yang, J. Photothermal and Photodynamic Therapies via NIR-Activated Nanoagents in Combating Alzheimer’s Disease. ACS Biomater. Sci. Eng. 2021, 7, 3573–3585. [Google Scholar] [CrossRef]
- Wang, M.; Dinarvand, D.; Chan, C.T.Y.; Bragin, A.; Li, L. Photobiomodulation as a Potential Treatment for Alzheimer’s Disease: A Review Paper. Brain Sci. 2024, 14, 1064. [Google Scholar] [CrossRef]
- Moorthy, H.; Datta, L.P.; Samanta, S.; Govindaraju, T. Multifunctional Architectures of Cyclic Dipeptide Copolymers and Composites, and Modulation of Multifaceted Amyloid-β Toxicity. ACS Appl. Mater. Interfaces 2022, 14, 56535–56547. [Google Scholar] [CrossRef]
- Li, M.; Guan, Y.; Zhao, A.; Ren, J.; Qu, X. Using Multifunctional Peptide Conjugated Au Nanorods for Monitoring β-amyloid Aggregation and Chemo-Photothermal Treatment of Alzheimer’s Disease. Theranostics 2017, 7, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Gao, N.; Dong, K.; Zhao, A.; Sun, H.; Wang, Y.; Ren, J.; Qu, X. Polyoxometalate-based nanozyme: Design of a multifunctional enzyme for multi-faceted treatment of Alzheimer’s disease. Nano Res. 2016, 9, 1079–1090. [Google Scholar] [CrossRef]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef]
- Chaudhary, H.; Iashchishyn, I.A.; Romanova, N.V.; Rambaran, M.A.; Musteikyte, G.; Smirnovas, V.; Holmboe, M.; Ohlin, C.A.; Svedružić, Z.M.; Morozova-Roche, L.A. Polyoxometalates as Effective Nano-inhibitors of Amyloid Aggregation of Pro-inflammatory S100A9 Protein Involved in Neurodegenerative Diseases. ACS Appl. Mater. Interfaces 2021, 13, 26721–26734. [Google Scholar] [CrossRef]
- Čolović, M.B.; Medić, B.; Ćetković, M.; Kravić Stevović, T.; Stojanović, M.; Ayass, W.W.; Ayass, W.W.; Mougharbel, A.S.; Radenković, M.; Prostran, M. Toxicity evaluation of two polyoxotungstates with anti-acetylcholinesterase activity. Toxicol. Appl. Pharmacol. 2017, 333, 68–75. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Lazarević-Pašti, T.D.; Leskovac, A.R.; Petrović, S.Ž.; Čolović, M.B.; Parac-Vogt, T.N.; Janjić, G.V. A new acetylcholinesterase allosteric site responsible for binding voluminous negatively charged molecules—The role in the mechanism of AChE inhibition. Eur. J. Pharm. Sci. 2020, 151, 105376. [Google Scholar] [CrossRef]
- Hua, J.; Wang, F.; Wei, X.; Qin, Y.; Lian, J.; Wu, J.; Ma, P.; Ma, X. A Nanoenzyme Constructed from Manganese and Strandberg-Type Phosphomolybdate with Versatility in Antioxidant and Modulating Conformation of Aβ Protein Misfolding Aggregates In Vitro. Int. J. Mol. Sci. 2023, 24, 4317. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.G.; Ruehl, C.L.; Morse, S.V.; Simon, M.; Rakers, V.; Watts, H.; Aprile, F.A.; Choi, J.J.; Vilar, R. Modulation of amyloid-β aggregation by metal complexes with a dual binding mode and their delivery across the blood–brain barrier using focused ultrasound. Chem. Sci. 2021, 12, 9485–9493. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, A.; Tyszka-Czochara, M.; Serda, P.; Oszajca, M.; Ruggiero-Mikołajczyk, M.; Pamin, K.; Napruszewska, B.D.; Prochownik, E.; Łasocha, W. New Organic-Inorganic Hybrid Compounds Based on Sodium Peroxidomolybdates (VI) and Derivatives of Pyridine Acids: Structure Determination and Catalytic Properties. Materials 2022, 15, 5976. [Google Scholar] [CrossRef]
- Pardiwala, A.; Kumar, S.; Jangir, R. Insights into organic–inorganic hybrid molecular materials: Organoimido functionalized polyoxomolybdates. Dalton Trans. 2022, 51, 4945–4975. [Google Scholar] [CrossRef]
- Narasimhan, K.; Micoine, K.; Lacôte, E.; Thorimbert, S.; Cheung, E.; Hasenknopf, B.; Jauch, R. Exploring the utility of organo-polyoxometalate hybrids to inhibit SOX transcription factors. Cell Regen. 2014, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Yang, E.; Kim, Y.; Zhao, Y.; Ma, W. Biomimetic Chiral Nanomaterials with Selective Catalysis Activity. Adv. Sci. 2024, 11, 2306979. [Google Scholar] [CrossRef]
- Jung, W.; Kwon, J.; Cho, W.; Yeom, J. Chiral Biomaterials for Nanomedicines: From Molecules to Supraparticles. Pharmaceutics 2022, 14, 1951. [Google Scholar] [CrossRef]
- Zhang, D.D.; Guo, Z.Y.; Guo, P.F.; Hu, X.; Chen, X.W.; Wang, J.H. Polyoxometalate-Coated Magnetic Nanospheres for Highly Selective Isolation of Immunoglobulin G. ACS Appl. Mater. Interfaces 2018, 10, 21876–21882. [Google Scholar] [CrossRef] [PubMed]
- Salazar Marcano, D.E.; Savić, N.D.; Abdelhameed, S.A.M.; De Azambuja, F.; Parac-Vogt, T.N. Exploring the Reactivity of Polyoxometalates toward Proteins: From Interactions to Mechanistic Insights. JACS Au 2023, 3, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; DeLaney, K.; Li, L. Molecular basis for chirality-regulated Aβ self-assembly and receptor recognition revealed by ion mobility-mass spectrometry. Nat. Commun. 2019, 10, 5038. [Google Scholar] [CrossRef]
- Serpell, L.C. Alzheimer’s amyloid fibrils: Structure and assembly. Biochim. Biophys. Acta Mol. Basis Dis. 2000, 1502, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding Post-Translational Modification Crosstalk with Proteomics. Mol. Cell. Proteom. 2021, 20, 100129. [Google Scholar] [CrossRef]
- Bludau, I.; Willems, S.; Zeng, W.F.; Strauss, M.T.; Hansen, F.M.; Tanzer, M.C.; Karayel, O.; Schulman, B.A.; Mann, M. The structural context of posttranslational modifications at a proteome-wide scale. PLoS Biol. 2022, 20, e3001636. [Google Scholar] [CrossRef]
- Lin, H.; Caroll, K.S. Introduction: Posttranslational Protein Modification. Chem. Rev. 2018, 118, 887–888. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiao, X.; Qiu, Y.; Xu, Z.; Chen, C.; Chong, B.; Zhao, X.; Hai, S.; An, Z.; Dai, L. Protein posttranslational modifications in health and diseases: Functions, regulatory mechanisms, and therapeutic implications. MedComm 2023, 4, e261. [Google Scholar] [CrossRef]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in cancer nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Artetxe, B.; Vivanco, M.M.; Gutiérrez-Zorrilla, J.M.; Vilas-Vilela, J.L. Chitosan nanogels as nanocarriers of polyoxometalates for breast cancer therapies. Carbohydr. Polym. 2019, 213, 159–167. [Google Scholar] [CrossRef]
- Zhou, R.; Liao, Y.; Xu, H.; Gu, Z.; Leng, Z. Polyoxometalates: Versatile nanomedicine candidates for precise cancer therapy. Chem. Eng. J. 2025, 512, 162335. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Zhang, X.; Li, P.; Wei, W.; Sun, S.; Chen, Y. Multifunctional natural chlorogenic acid based nanocarrier for Alzheimer’s disease treatment. Mater. Today Bio 2025, 32, 101841. [Google Scholar] [CrossRef]
- Wang, M.; Yan, C.; Li, X.; Yang, T.; Wu, S.; Liu, Q.; Luo, Q.; Zhou, F. Non-invasive modulation of meningeal lymphatics ameliorates ageing and Alzheimer’s disease-associated pathology and cognition in mice. Nat. Commun. 2024, 15, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, Y.; Cheng, X.; Xie, X.; Zeng, Y.; Tao, Q.; Yang, Y.; Xiao, C.; Zhang, Z.; Pang, J. Modulation of glymphatic system by visual circuit activation alleviates memory impairment and apathy in a mouse model of Alzheimer’s disease. Nat. Commun. 2025, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.P.; Zhang, S.; Zhao, Y.; Ge, X.Y.; Yang, L.; Li, K.; Feng, L.B.; Li, R.G.; Chen, J.J. Tunable multi-electron redox polyoxometalates for decoupled water splitting driven by sunlight. Nat. Commun. 2025, 16, 3674. [Google Scholar] [CrossRef]
- Fu, M.; Han, Y.; Nie, Y.; Liu, Y.; Liu, Y.; He, P.; Yu, W.D.; Li, X.; He, P.; Li, J. Photothermal conversion property studies of polyoxophosphitemolybdate derivatives through microwave-assisted synthesis. Rare Met. 2024, 43, 6023–6033. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Hou, L.; Geng, W.; Wang, J.; Kong, Y.; Liu, C.; Zeng, X.; Kong, D. A Biomimetic Upconversion Nanobait-Based Near Infrared Light Guided Photodynamic Therapy Alleviates Alzheimer’s Disease by Inhibiting β-Amyloid Aggregation. Adv. Healthc. Mater. 2024, 13, 2303278. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Yang, J.; Zhang, L.; Yan, J. An in situ -activated and chemi-excited photooxygenation system based on G-poly(thioacetal) for Aβ1–42 aggregates. J. Mater. Chem. B 2024, 12, 10850–10860. [Google Scholar] [CrossRef]
- Pass, H.I. Photodynamic Therapy in Oncology: Mechanisms and Clinical Use. J. Natl. Cancer Inst. 1993, 85, 443–456. [Google Scholar] [CrossRef]
- Gao, F.; Hou, Y.; Wang, Y.; Liu, L.; Yi, X.; Xia, N. Photothermal and Photodynamic Strategies for Diagnosis and Therapy of Alzheimer’s Disease by Modulating Amyloid-β Aggregation. Biosensors 2025, 15, 480. [Google Scholar] [CrossRef]
- Yu, L.; Ding, Y.; Zheng, M. Polyoxometalate-based manganese clusters as catalysts for efficient photocatalytic and electrochemical water oxidation. Appl. Catal. B Environ. 2017, 209, 45–52. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Sun, J.; Zhi, Y.; Xie, Z.; Guan, Y.; Zhang, Z.; Wu, C. Photothermal nano-agents: An innovative trident weapon for accurate and effective treatment of Alzheimer’s disease. Nanobiotechnol 2025, 23, 650. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Xie, W.; Sun, J.; Yang, L.; Liu, J. Penetratin Peptide-Functionalized Gold Nanostars: Enhanced BBB Permeability and NIR Photothermal Treatment of Alzheimer’s Disease Using Ultralow Irradiance. ACS Appl. Mater. Interfaces 2016, 8, 19291–19302. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Zhang, Y.; Pan, J.; Wang, K.; Wang, H.X. Near-infrared irradiation controlled thermo-switchable polymeric photosensitizer against β-amyloid fibrillation. J. Mater. Chem. B 2022, 10, 4832–4839. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.S.; Nozdriukhin, D.; Chuprov-Netochin, R.; Tsydenzhapova, E.; Novoselova, M.; Gorin, D.; Yashchenok, A. Engineered multicompartment vesicosomes for selective uptake by living cells. Colloids Surf. B Biointerfaces 2022, 220, 112953. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, D.; Wang, J.; Li, J.; Pang, X.; Shan, H.; Zhang, K. Injectable and NIR-responsive CDN–POM hydrogels for combined non-inflammatory photo-immunotherapy. J. Mater. Chem. B 2024, 12, 8616–8625. [Google Scholar] [CrossRef]
- Ma, M.; Qu, X. Recent Advances on Polyoxometalates as Metallodrug Agents for Alzheimer’s Disease. Chem. J. Chin. Univ. 2020, 41, 884–891. [Google Scholar]
- Abdelhameed, S.A.M.; Ly, H.G.T.; Moons, J.; De Azambuja, F.; Proost, P.; Parac-Vogt, T.N. Expanding the reactivity of inorganic clusters towards proteins: The interplay between the redox and hydrolytic activity of Ce(IV)-substituted polyoxometalates as artificial proteases. Chem. Sci. 2021, 12, 10655–10663. [Google Scholar] [CrossRef]
- Salazar Marcano, D.E.; Parac-Vogt, T.N. Hybrid functional materials merging polyoxometalates and biomolecules: From synthesis to applications. Coord. Chem. Rev. 2024, 518, 216086. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Savić, N.D.; Rodriguez, A.; Parac-Vogt, T.N. Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates. Molecules 2020, 25, 3472. [Google Scholar] [CrossRef]
- Sap, A.; Absillis, G.; Parac-Vogt, T.N. Selective hydrolysis of oxidized insulin chain B by a Zr(IV)-substituted Wells–Dawson polyoxometalate. Dalton Trans. 2015, 44, 1539–1548. [Google Scholar] [CrossRef]
- Abdelhameed, S.A.M.; Vandebroek, L.; De Azambuja, F.; Parac-Vogt, T.N. Redox Activity of Ce(IV)-Substituted Polyoxometalates toward Amino Acids and Peptides. Inorg. Chem. 2020, 59, 10569–10577. [Google Scholar] [CrossRef]
- Absillis, G.; Parac-Vogt, T.N. Peptide Bond Hydrolysis Catalyzed by the Wells–Dawson Zr(α2-P2W17O61)2 Polyoxometalate. Inorg. Chem. 2012, 51, 9902–9910. [Google Scholar] [CrossRef]
- Stroobants, K.; Absillis, G.; Moelants, E.; Proost, P.; Parac-Vogt, T.N. Regioselective Hydrolysis of Human Serum Albumin by ZrIV -Substituted Polyoxotungstates at the Interface of Positively Charged Protein Surface Patches and Negatively Charged Amino Acid Residues. Chem. Eur. J. 2014, 20, 3894–3897. [Google Scholar] [CrossRef]
- Anyushin Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography—An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef]
- Sap Nasb, M.; Tao, W.; Chen, M. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar]
- Saku, Y.; Sakai, Y.; Nomiya, K. Relation among the 2:2-, 1:1- and 1:2-type complexes of hafnium(IV)/zirconium(IV) with mono-lacunary α2-Dawson polyoxometalate ligands: Synthesis and structure of the 2:2-type complexes [{α2-P2W17O61M(μ-OH)(H2O)}2]14− (M = Hf, Zr). Inorg. Chim. Acta 2010, 363, 967–974. [Google Scholar] [CrossRef]
- Bandakinda, M.; Mishra, A. Insights into role of microRNA in Alzheimer’s disease: From contemporary research to bedside perspective. Int. J. Biol. Macromol. 2023, 253, 126561. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Li, Y.; Chen, Y.; Wang, X.; Zang, D.; Liu, T. Polyoxometalate-based nanocomposites for antitumor and antibacterial applications. Nanoscale Adv. 2022, 4, 3689–3706. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef]
- Pampuscenko, K.; Jankeviciute, S.; Morkuniene, R.; Sulskis, D.; Smirnovas, V.; Brown, G.C.; Borutaite, V. S100A9 protein activates microglia and stimulates phagocytosis, resulting in synaptic and neuronal loss. Neurobiol. Dis. 2025, 206, 106817. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Iashchishyn, I.A.; Pansieri, J.; Nyström, S.; Klementieva, O.; Kara, J.; Horvath, I.; Moskalenko, R.; Rofougaran, R.; Gouras, G. S100A9-Driven Amyloid-Neuroinflammatory Cascade in Traumatic Brain Injury as a Precursor State for Alzheimer’s Disease. Sci. Rep. 2018, 8, 12836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Gilthorpe, J.; Van Der Maarel, J.R.C. MRP14 (S100A9) Protein Interacts with Alzheimer Beta-Amyloid Peptide and Induces Its Fibrillization. PLoS ONE 2012, 7, e32953. [Google Scholar] [CrossRef]
- Horvath, I.; Iashchishyn, I.A.; Moskalenko, R.A.; Wang, C.; Wärmländer, S.K.T.S.; Wallin, C.; Gräslund, A.; Kovacs, G.G.; Morozova-Roche, L.A. Co-aggregation of pro-inflammatory S100A9 with α-synuclein in Parkinson’s disease: Ex vivo and in vitro studies. J. Neuroinflamm. 2018, 15, 172. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimers Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Terry, A.V.; Buccafusco, J.J. The Cholinergic Hypothesis of Age and Alzheimer’s Disease-Related Cognitive Deficits: Recent Challenges and Their Implications for Novel Drug Development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Hartmann, J.; Kiewert, C.; Duysen, E.G.; Lockridge, O.; Greig, N.H.; Klein, J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 2007, 100, 1421–1429. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.; Khan, J.; Kamal, M. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Zuin, M.; Cherubini, A.; Volpato, S.; Ferrucci, L.; Zuliani, G. Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia. Sci. Rep. 2022, 12, 12214. [Google Scholar] [CrossRef] [PubMed]
- Furukawa-Hibi, Y.; Alkam, T.; Nitta, A.; Matsuyama, A.; Mizoguchi, H.; Suzuki, K.; Moussaoui, S.; Yu, Q.S.; Greig, N.H.; Nagai, T. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-β peptide in mice. Behav. Brain Res. 2011, 225, 222–229. [Google Scholar] [CrossRef]
- Žužek, M.C. Advances in Cholinesterase Inhibitor Research—An Overview of Preclinical Studies of Selected Organoruthenium(II) Complexes. Int. J. Mol. Sci. 2024, 25, 9049. [Google Scholar] [CrossRef]
- Holt, K.; Payne, E.; Spires-Jones, T.L. Not all plaques are created equal: Uncovering a unique molecular signature in Alzheimer’s disease. Brain Neurosci. Adv. 2024, 8, 23982128241280001. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between polyoxometalates and biological systems: From drug design to artificial enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, S.; Zhao, C.; Li, X.; Jin, J.; Shi, X.; Su, Z.; Li, J.; Wang, J. Promising application of polyoxometalates in the treatment of cancer, infectious diseases and Alzheimer’s disease. J. Biol. Inorg. Chem. 2022, 27, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Das, U. Generative AI for drug discovery and protein design: The next frontier in AI-driven molecular science. Med. Drug Discov. 2025, 27, 100213. [Google Scholar] [CrossRef]
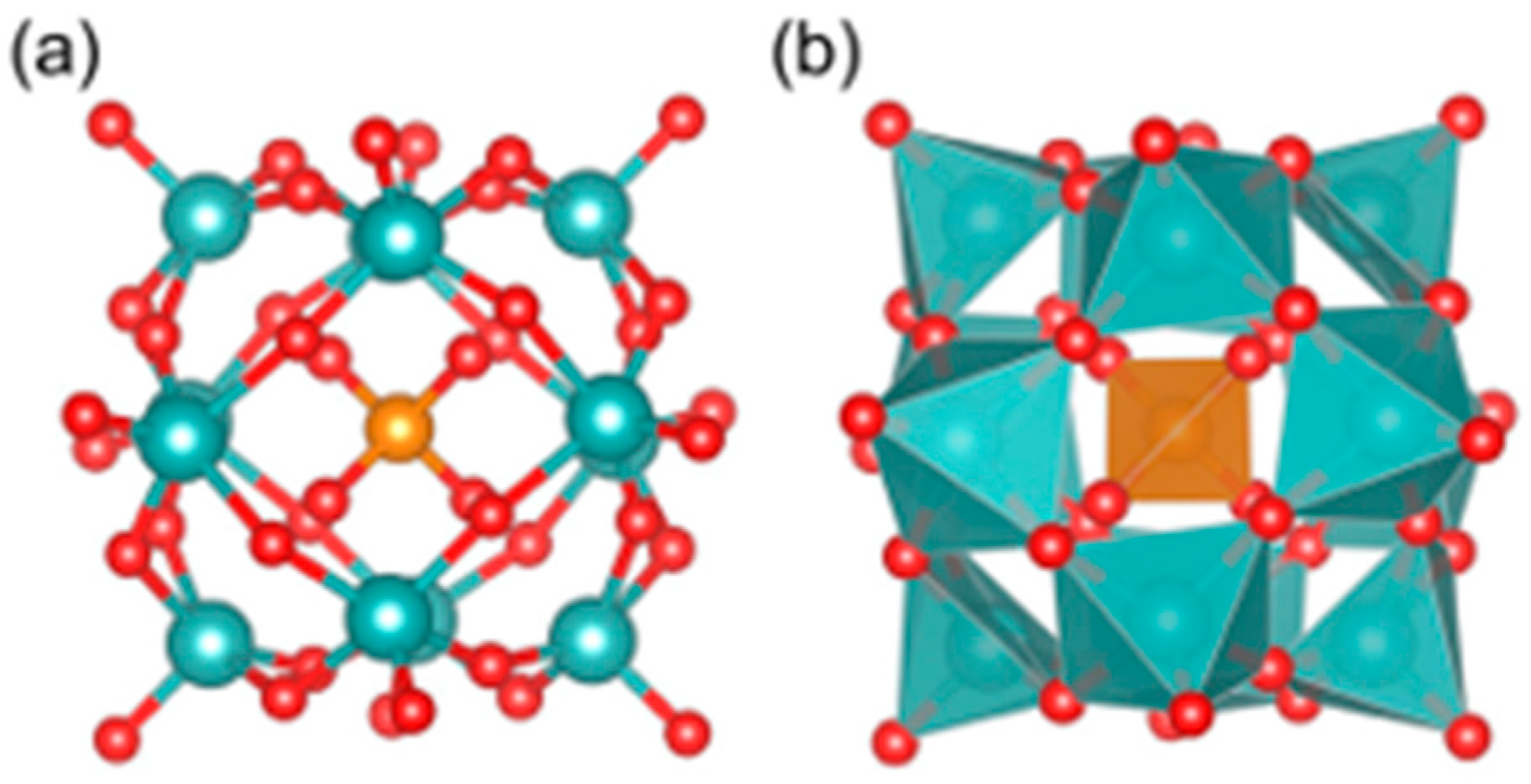
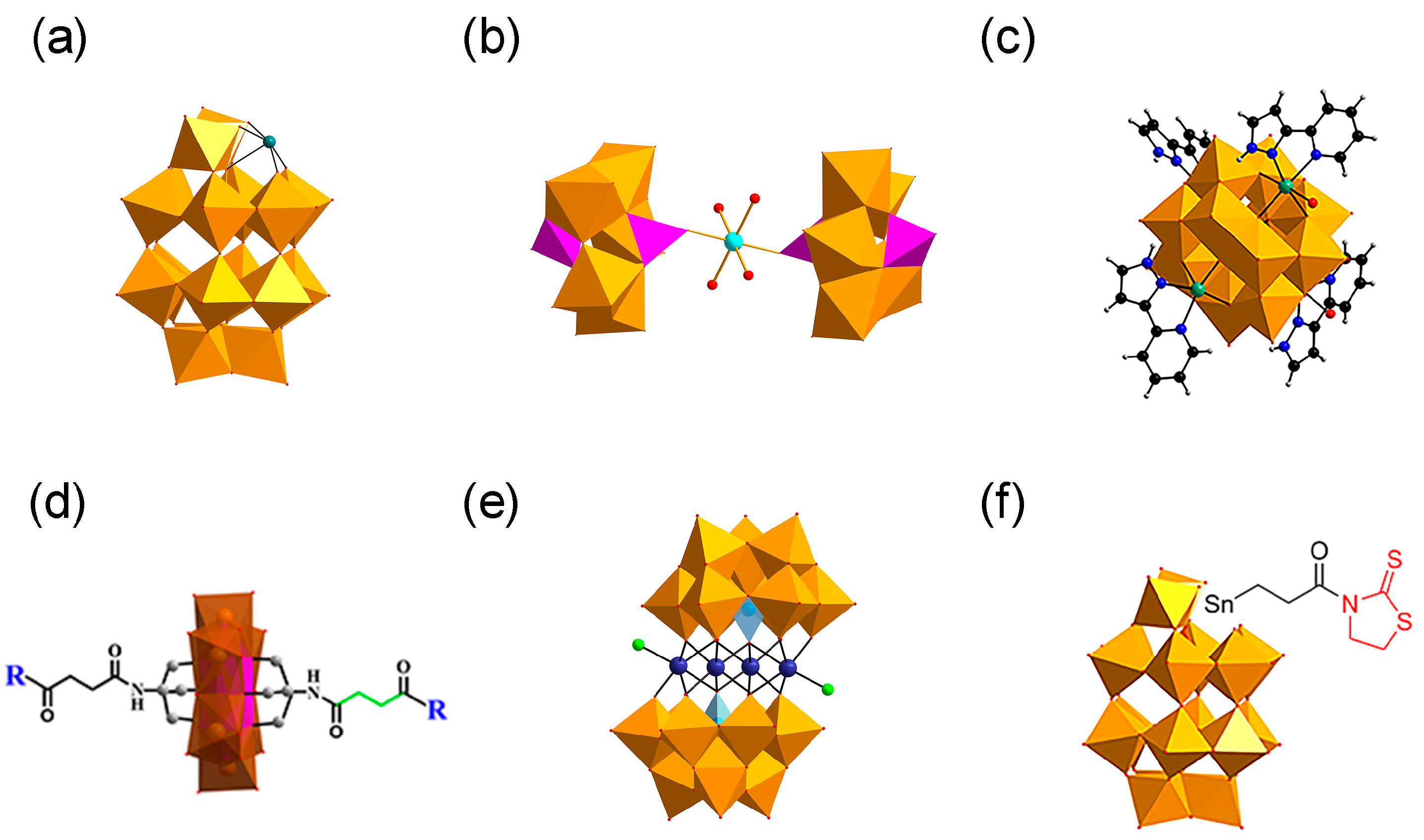
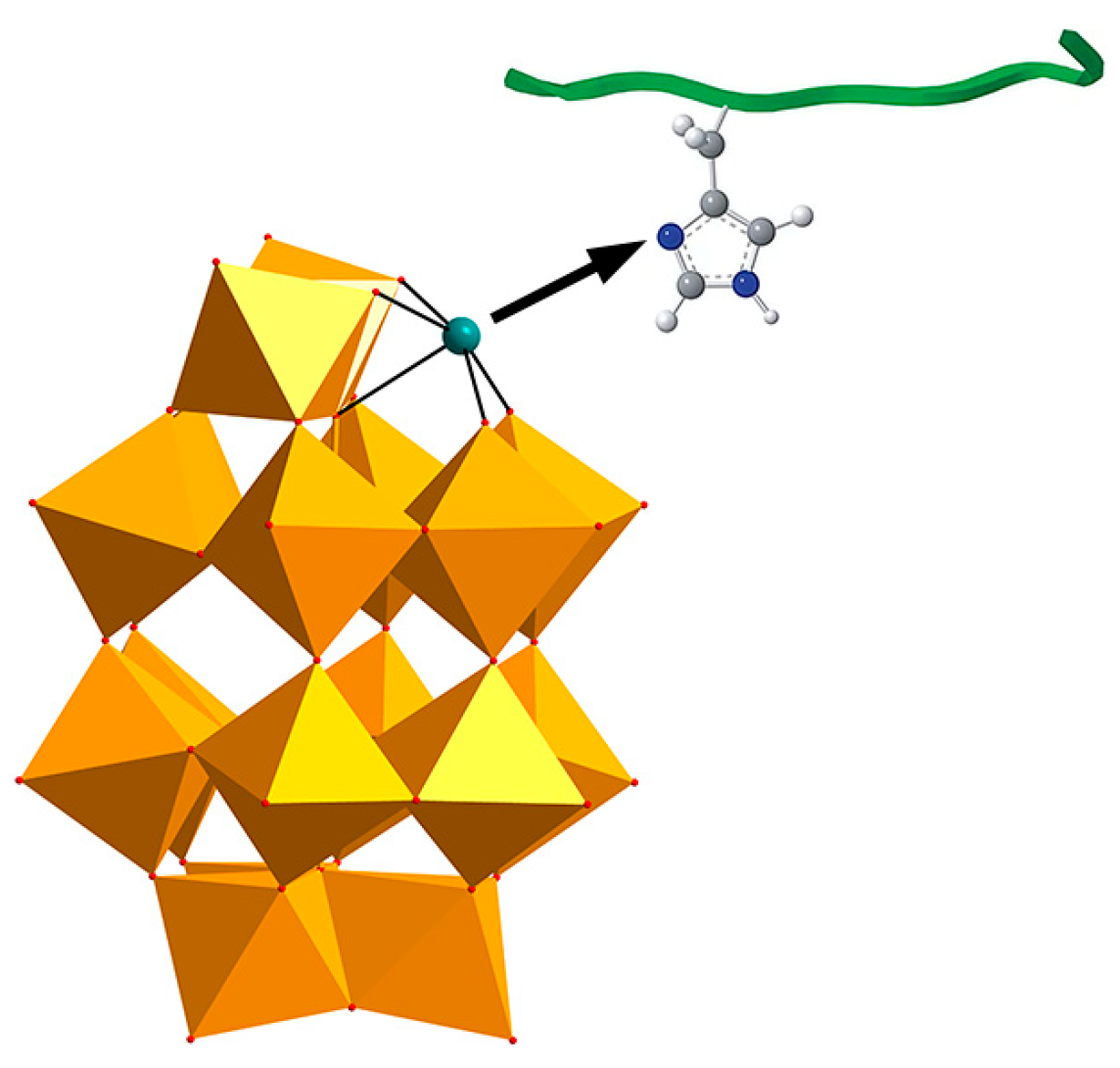
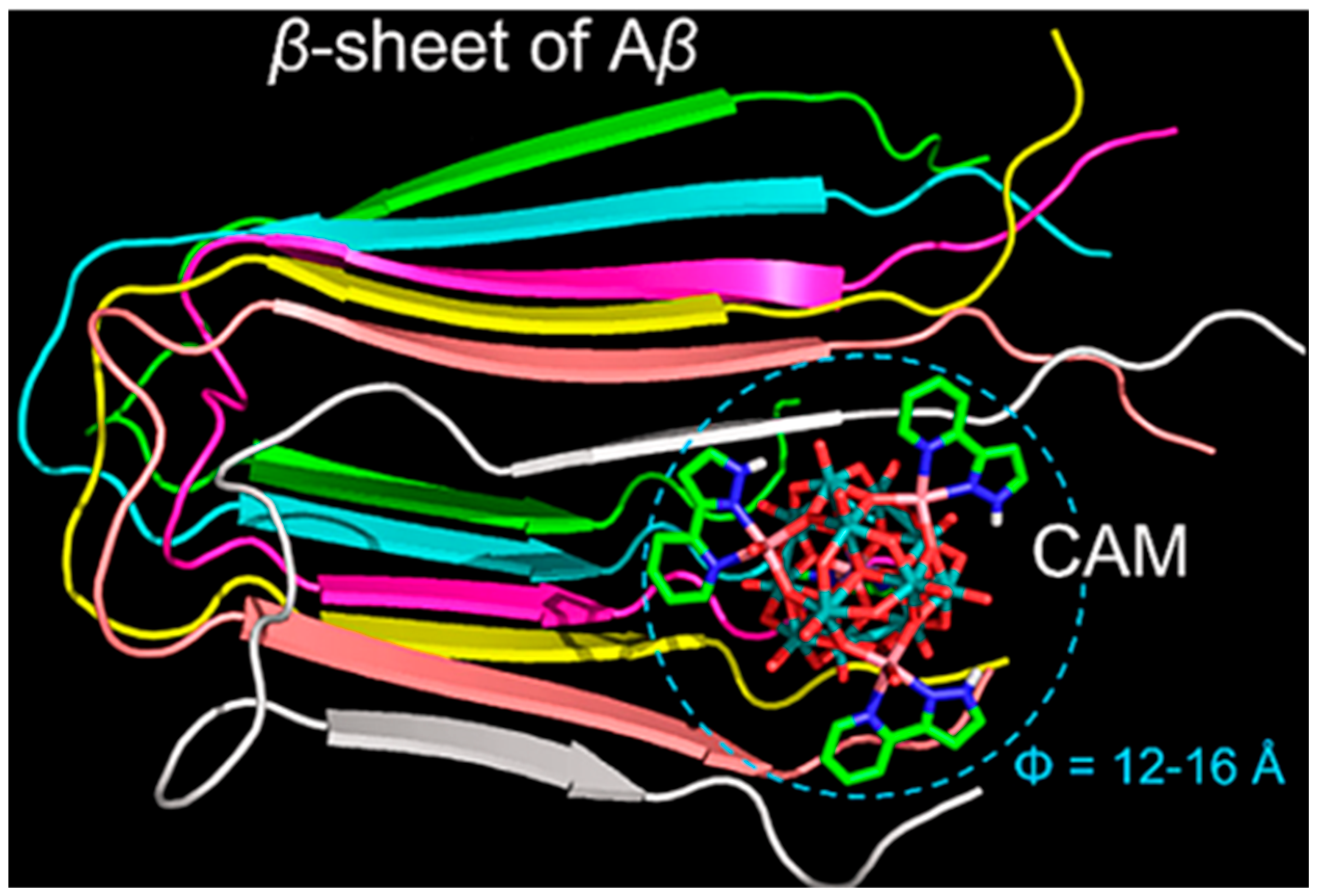
| Category | Protein Aggregates | Name | Molecular Mechanism of Action | Quantitative Data | Multi-Target Effects | Ref. |
|---|---|---|---|---|---|---|
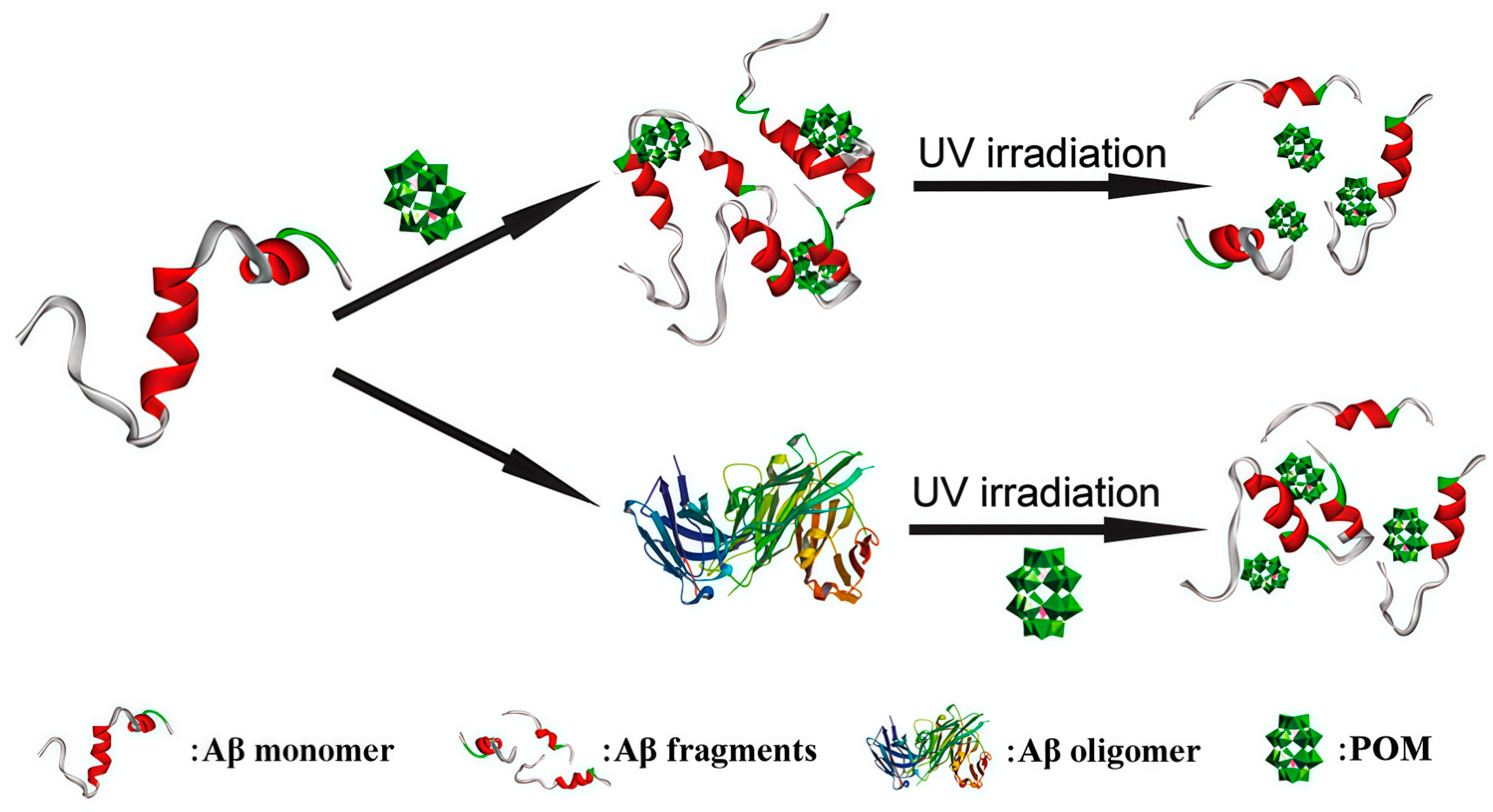
| 1 | Aβ | K8[P2CoW17O61] | Binds to Aβ through electrostatic interactions, generates ROS upon light excitation, and disrupts Aβ hydrogen bonds and secondary structure. | DCF fluorescence intensity increased by 163% (8 μM). | [32,46] | |
| 2 | Aβ | Na9H[SiW9O34], K7[PTi2W10O40], K8[β-SiW11O39] | POMs binds to the His13-Lys16 cationic region of Aβ through electrostatic interactions, thereby inhibiting further Aβ aggregation. | Na9H[SiW9O34]: Aβ inhibition IC50 = 19.85 μM (in vitro, ThT); K7[PTi2W10O40]: Aβ inhibition IC50 = 39.04 μM (in vitro, ThT); K8[β-SiW11O39]: Aβ inhibition IC50 = 39.02 μM (in vitro, ThT). | [32] | |
| 3 | Aβ | (NH4)42{Mo132O372(OAc)30} * | By binding through negative charges to the positively charged His13-Lys16 region of Aβ, and simultaneously chelating Zn2+/Cu2+ to reduce their concentration, it achieves dual inhibition of Aβ aggregation. | ROS reduction: 34% (10 μg/mL); Cell viability: >50% (10 μg/mL). | Inhibition of Aβ aggregation + metal ion chelation | [47] |
| 4 | Aβ | [PO40Mo12]3− | Inhibition of Aβ fibrils formation via multi-weak interactions (H-bonds, van der Waals). | Binding energy: −1.1 to −1.6 eV, ≈ −5 eV; Energy difference: ≥1–3 eV; Orbital gap: ≈ 2.1 eV (P–Iso/Gly/Leu), average 1.5 eV (P-Met). | [48] | |
| 5 | Aβ | K8[P2NiW17O61], K8[P2CoW17O61] | Inhibition of Aβ fibrils formation via electrostatic attraction and His-chelating effect. | Aβ aggregation inhibition IC50: Ni-POM 5.67 ± 1.53 μM, Co-POM 14.73 ± 2.37 μM; BBB penetration: Brain W peak 0.0231 mg/kg (Ni-POM, 25 mg/kg iv); In vivo metabolism: Returns to initial concentration at 48 h (Ni-POM); Self-cytotoxicity: No significant toxicity (≤120 μM). | [15] | |
| 6 | Aβ | K8[Co(H2O)4[HP2Mo5O23]2 | POM blocks Aβ β-sheet folding via Co2+ coordination with nitrogen heterocycles and its oxygen-rich surface. | [49] | ||
| 7 | Aβ | {[CoL-(H2O)]2[CoL]2[HAsVMoVI6O40]} | Target Aβ’s β-sheet via L group; inhibit/disaggregate Aβ via H-bonds and spatial embedding. | ROS scavenging: Reduced by 50% (Cu2+-Aβ induced); Cell viability: >40% (12 μM CAM); BBB penetration: Brain Mo peak ≈ 0.25 mg/kg (80 mg/kg iv); In vivo toxicity: No adverse effects (80 mg/kg). | [50] | |
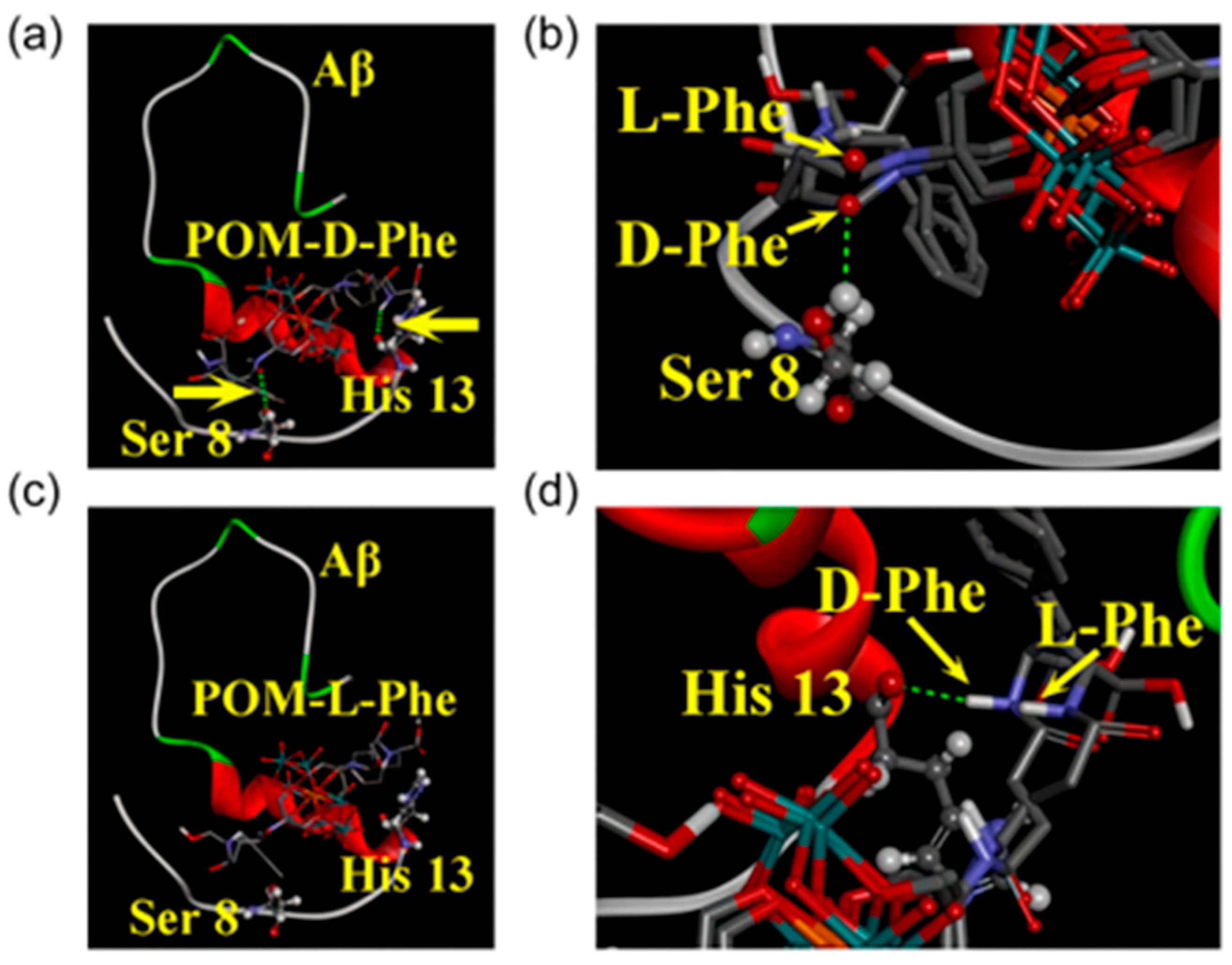
| 8 | Aβ | [MnMo9]-D-Phe, [MnMo9]-L-Phe | Bind to the α/β discordant segment of Aβ via hydrophobic interactions, π–π stacking, electrostatic interactions, and hydrogen bonds, disrupting its aggregation-related effects. | ROS scavenging: D-Phe-modified 106%; L-Phe-modified 116% (10 μM); BBB penetration: 1.98% (D-Phe-modified, 25 mg/kg iv); High-concentration toxicity: No significant toxicity (320 μM). | [35] | |
| 9 | Aβ | (Me4N)3[PW11O40(SiC3H6NH2)2PtCl2] * | Pt2+ coordinates with Aβ42 amino acids and binds the HHQK cationic cluster via electrostatics, hydrogen bonding, van der Waals forces, and π–π stacking, inhibiting aggregation; its planar conformation disrupts fibrils and promotes degradation. | IC50: 0.62 μM; β-sheet reduction: 45.3 ± 0.9% → 41.4 ± 1.1% (8 μM)/41.2 ± 1.5% (16 μM); Fibril disassembly: 33–92% (10–100 µM) Cell viability (PC12): 49% → 67% (8 μM); In vivo (1.5 mg/mL): Passive avoidance latency 21.57 s → 76.74 s. | [51] | |
| 10 | Aβ | (H2dap)6[Cd4Cl2(B-α-AsW9O34)2] | Surface O atoms form H-bonds with Aβ; Cd2+ coordinates His residues and competes with Zn2+/Cu2+, inhibiting misfolding and β-sheet aggregation. | ROS production decreased by 50% (in the Cu2+-β system, 20 µM); cell viability improved from <30% to >60%. | [52] | |
| 11 | Aβ | POMD-TZ | Site-selectively modifies Aβ and binds its cationic region to inhibit Aβ aggregation. | BBB penetration: ~1.59% (brain W conc. 0.256 mg/kg, delayed by ~10 min) | [53] | |
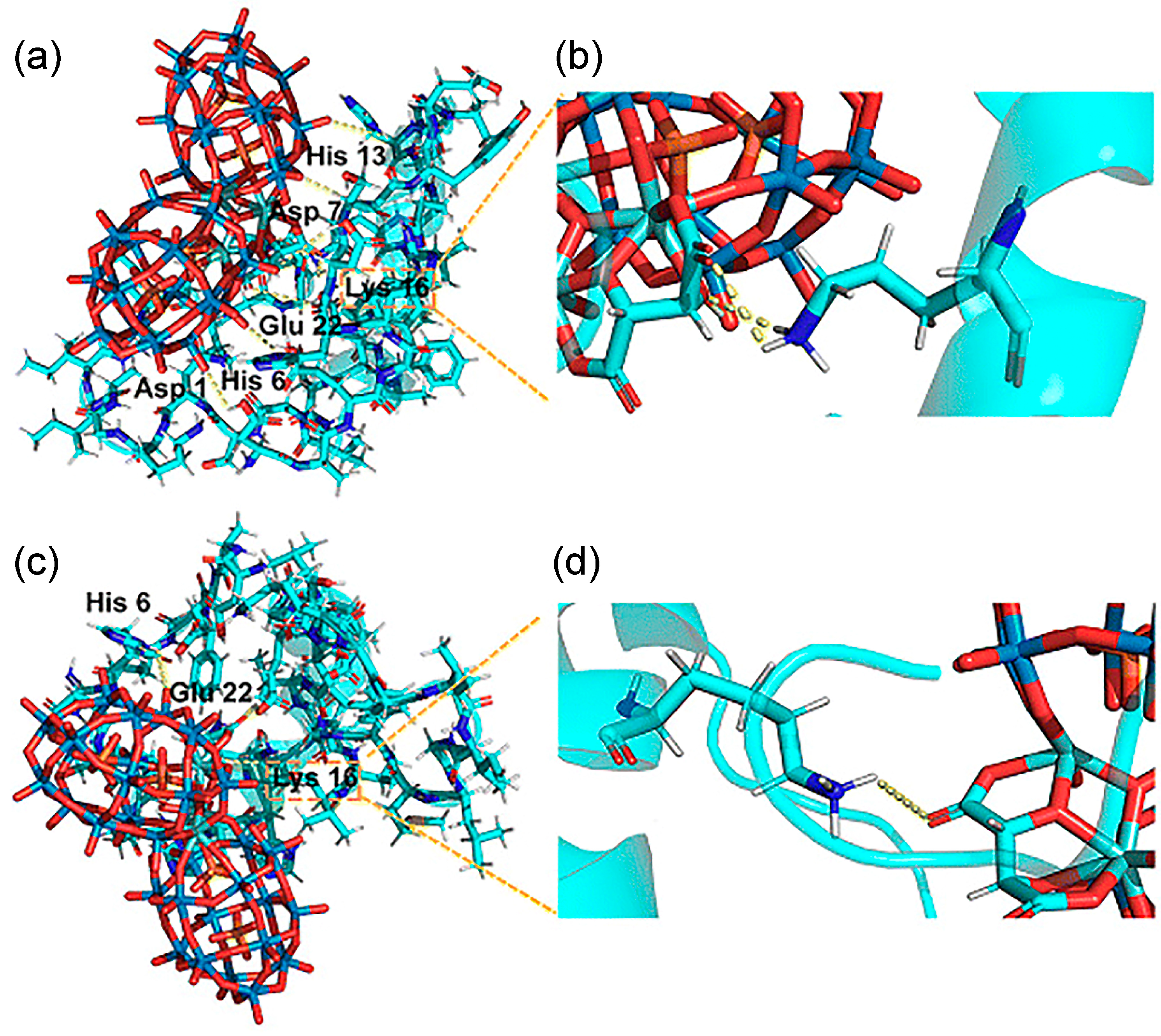
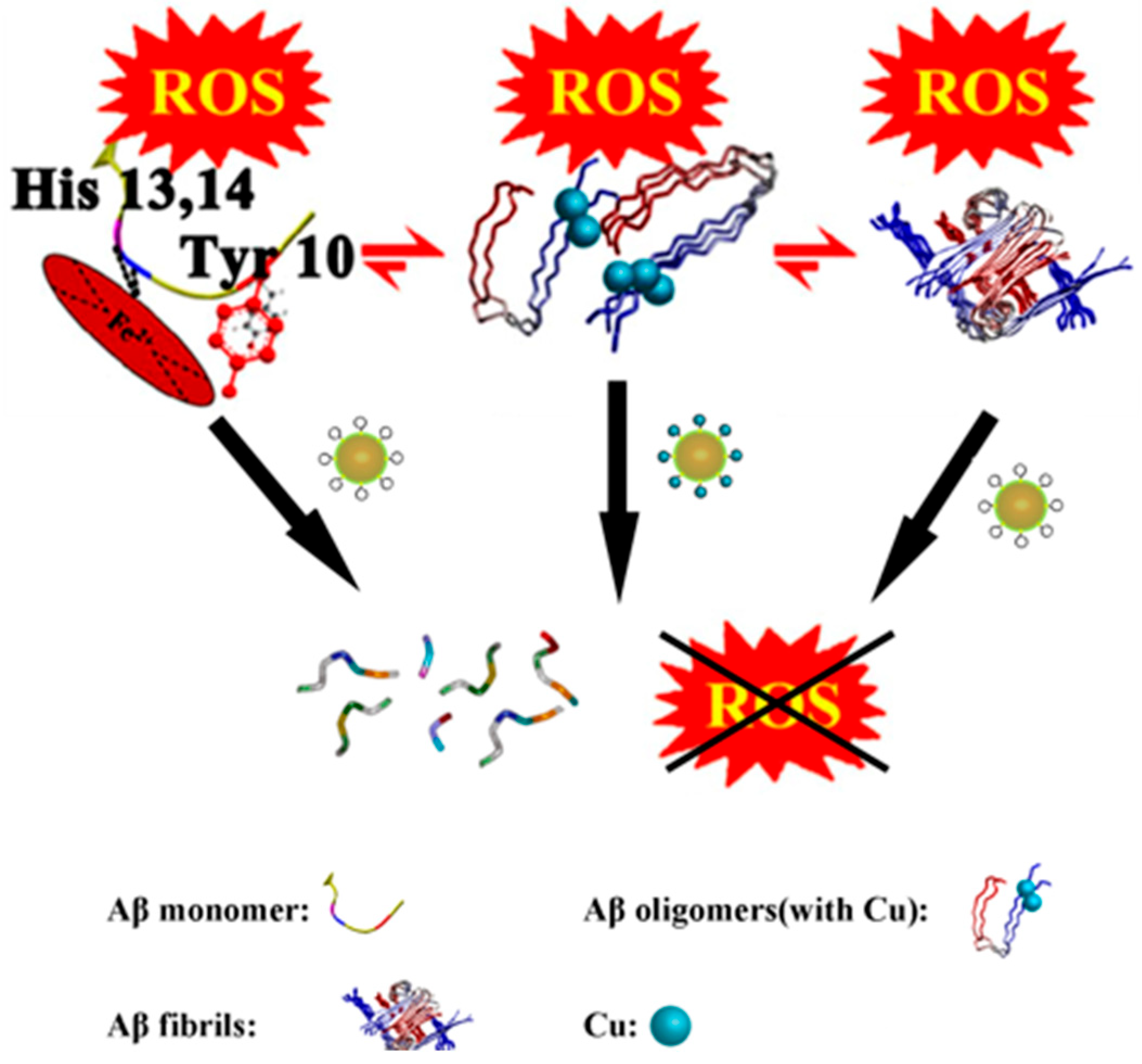
| 12 | Aβ | [(CH3)2NH2]15{α-P2W15Zr3(L-tartH)[αP2W16]} *, [(CH3)2NH2]15{α-P2W15Zr3(D-tartH) [α-P2W16]} * | Binds the HHQK cationic cluster of Aβ via electrostatic interactions; L/D-tartrate forms hydrogen bonds with Aβ, inhibiting aggregation; W-containing structure scavenges ROS via W5+/W6+ redox cycling. | Aβ aggregation inhibition: L-POM 43.90%, D-POM 26.45%; IC50: L-POM 17.38 μM, D-POM 2.63 μM; Binding constants: D-POM 1.07 × 106 M−1, L-POM 5.40 × 105 M−1 ROS scavenging: ~70% at 50 μM (both); PC12: Aβ-induced ROS 143%/viability 42%; Brain biodistribution: L-POM peak at 4 h, D-POM at 6 h. | Inhibit Aβ aggregation + scavenge ROS | [36] |
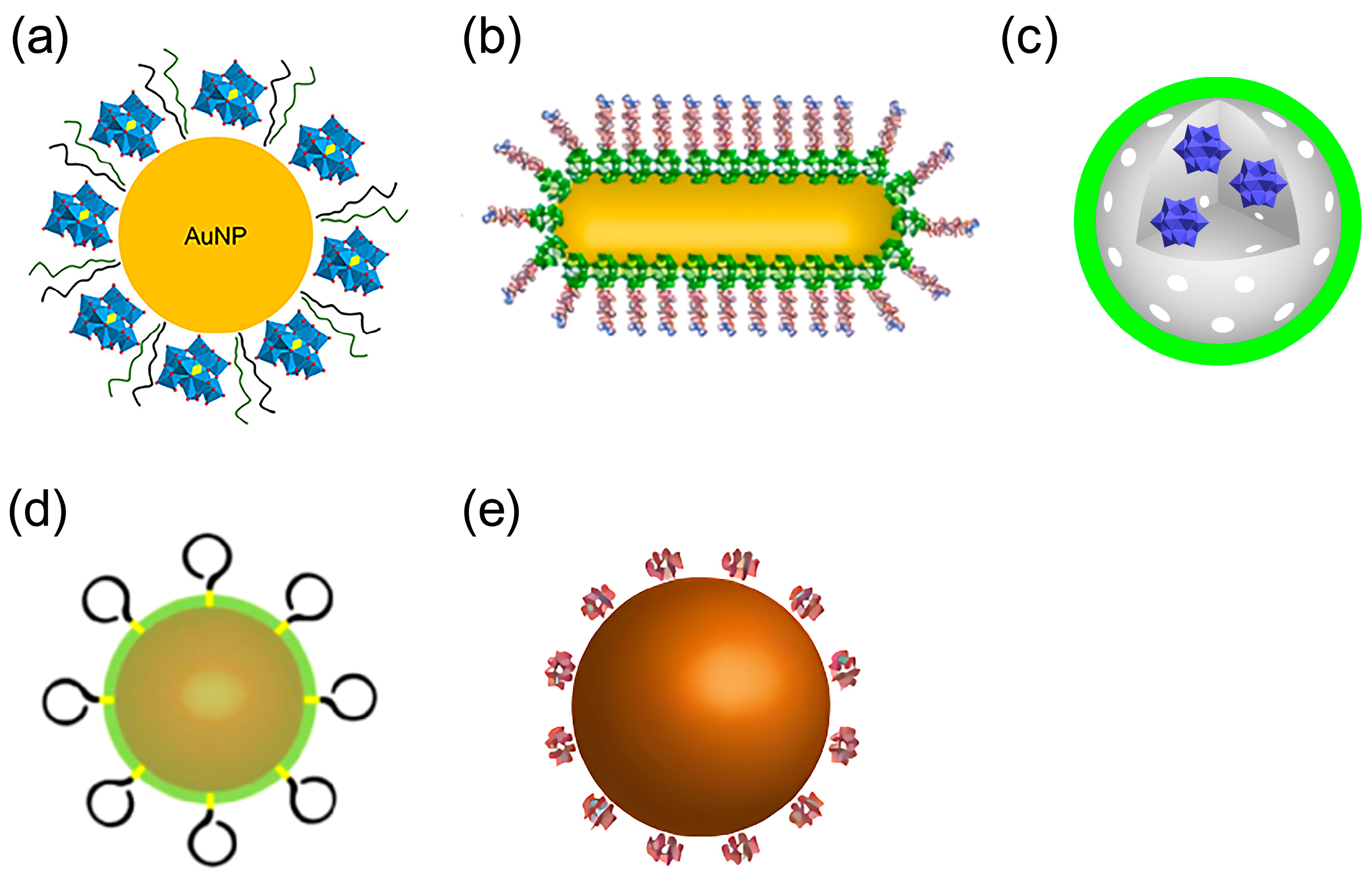
| 13 | Aβ | POM@P | The negatively charged surface of POM@P binds Aβ cationic clusters, increasing local peptide density, while the released POMs synergistically inhibit Aβ aggregation. | Aβ aggregation inhibition effect: ThT fluorescence inhibition rate over 65%; Cell protection effect: Cell viability increased to 82% (6 μM). | [54] | |
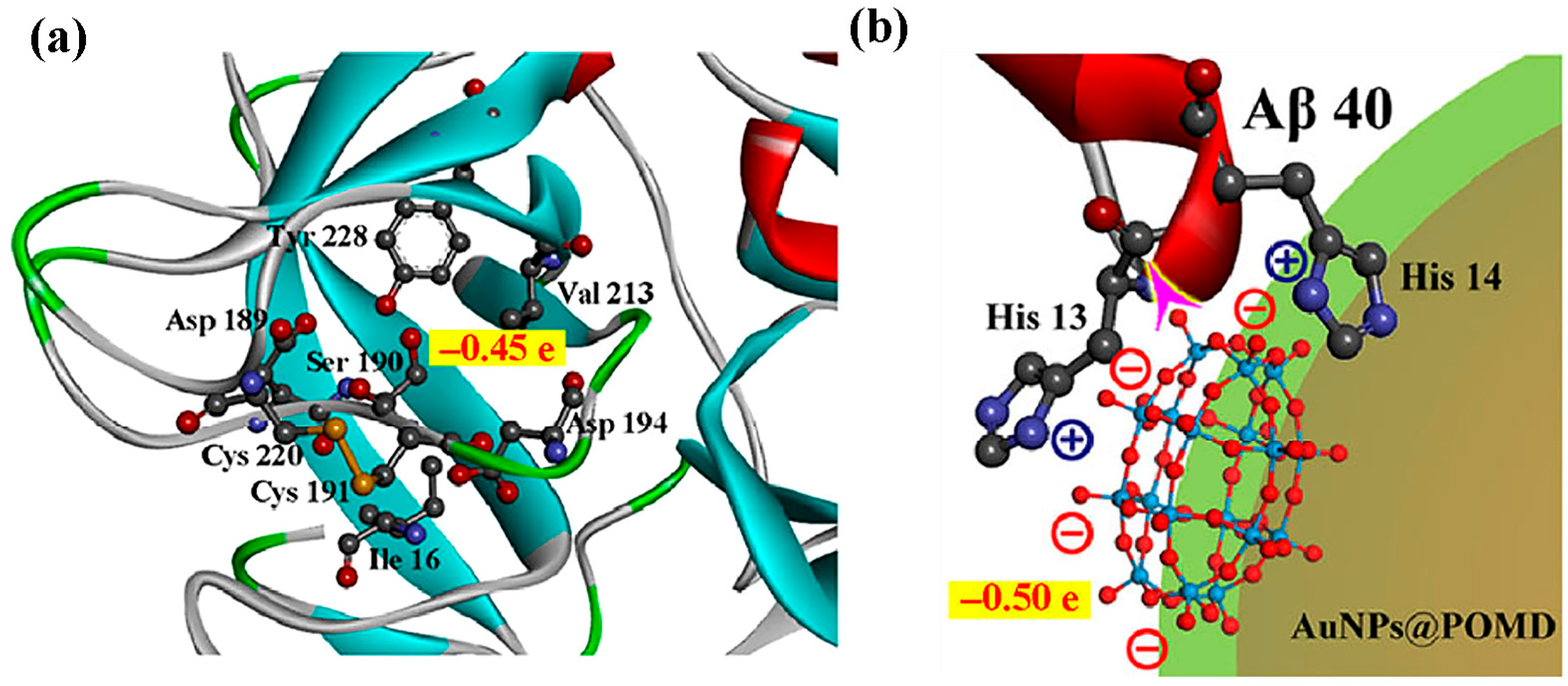
| 14 | Aβ | AuNPs@POMD-pep | POMD binds Aβ via electrostatic and hydrogen-bond interactions, while the peptide segment binds Aβ through hydrophobic interactions, synergistically inhibiting aggregation and dissociating fibrils. | Aβ aggregation inhibition rate (40 nm): 47%; Aβ fibril dissociation rate (40 nm): 37%; Aβ-mediated peroxidase activity inhibition rate (1 nm): 63%; Cell survival rate (5 nm): >90%; IC50 ratio (vs. N-Ac-CLPFFD): 1/6.14 (6.14x lower); IC50 ratio (vs. AuNPs@POMD): 1/4.31 (4.31x lower). | [55] | |
| 15 | Aβ | Peptide@Mo-POMs * | Peptide blocks Zn2+ sites on Aβ; Mo-POMs chelate Zn2+, synergistically inhibiting aggregation and disrupting protofibrils. | Apoptosis rate: 58.8% → 28.0%; Cell viability: 38.9% → >75%; ROS level: +107.9–38.6%. | Chelation of Zn2+ + Inhibition of Aβ Aggregation + Disassembly of Aβ Protofibrils | [37] |
| 16 | Aβ | CP-POM * | CP-POM inhibits Aβ42 aggregation and dissolves pre-aggregates through hydrogen bonding, hydrophobic interactions, and metal chelation. | Aβ42 fibril inhibition: ~45% (500 nM); Aβ42 fibril inhibition: 75% (100 nM); Aβ42 oligomer inhibition: 85% (100 nM); Preformed Aβ42 aggregate dissolution: 50% (100 nM); Neuronal viability improvement: ~64% (500 nM). | Inhibition of Aβ42 aggregation, dissolution of Aβ42 oligomers, metal chelation, and ROS scavenging | [56] |
| 17 | Aβ | AuNPs@POM@PEG | Inhibit Aβ aggregation via POM-Aβ binding. | Aβ inhibition: 75% (in vitro); Non-cytotoxic: <2.5 nM (neurovascular cells); BBB Pe: 3.47 vs. 3.07 × 10−6 cm/s. | [38] | |
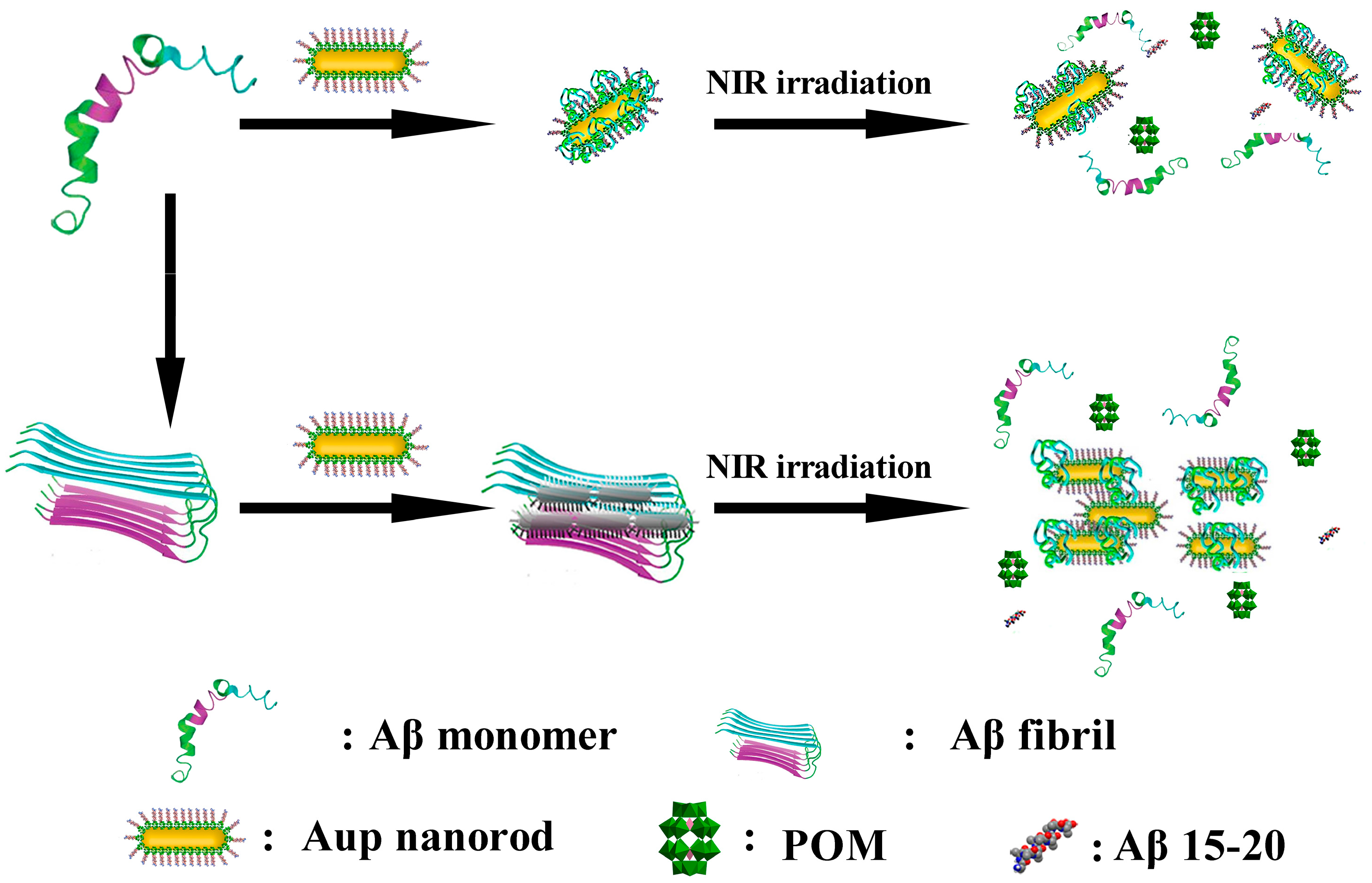
| 18 | Aβ | AuP | Aβ15-20 targeting → POMs/peptide inhibition → NIR photothermal Aβ fibril disassembly | BBB Penetration: 2.097 ± 0.337% (Brain Au accumulation); PC12 viability ↓ ~46% (Aβ 5μM). | [57] | |
| 19 | Aβ | rPOMDs@MSNs@ copolymers * | Under NIR laser: generates local hyperthermia to disaggregate Aβ fibrils; releases rPOMDs to inhibit Aβ aggregation. | Aβ fibril disaggregation: ThT ↓ 32.7% (with NIR); Aβ aggregation inhibition: ThT ↓ 74.7%; turbidity 0.024; ROS scavenging: ↓ 52.8%; Cytoprotection: Cell viability ↑ 92.6% (Aβ + NIR). | Inhibit Aβ + Disaggregate preformed Aβfibrils + Scavenge ROS | [58] |
| 20 | Aβ | AuNPs@POMD-8pep * | Inhibits Aβ aggregation via electrostatic and sequence-specific binding, cleaves fibrils through histidine-mediated protease-like activity, scavenges ROS via redox-active SOD-like sites, and chelates Cu2+ to block metal-induced Aβ aggregation. | Protease specific activity: (8.80 ± 0.32) × 105 U·mg−1; BBB penetration: Brain concentration peaks at 1 h post-administration. | Inhibit Aβ + Hydrolyze Aβ + chelate Cu2+ | [59] |
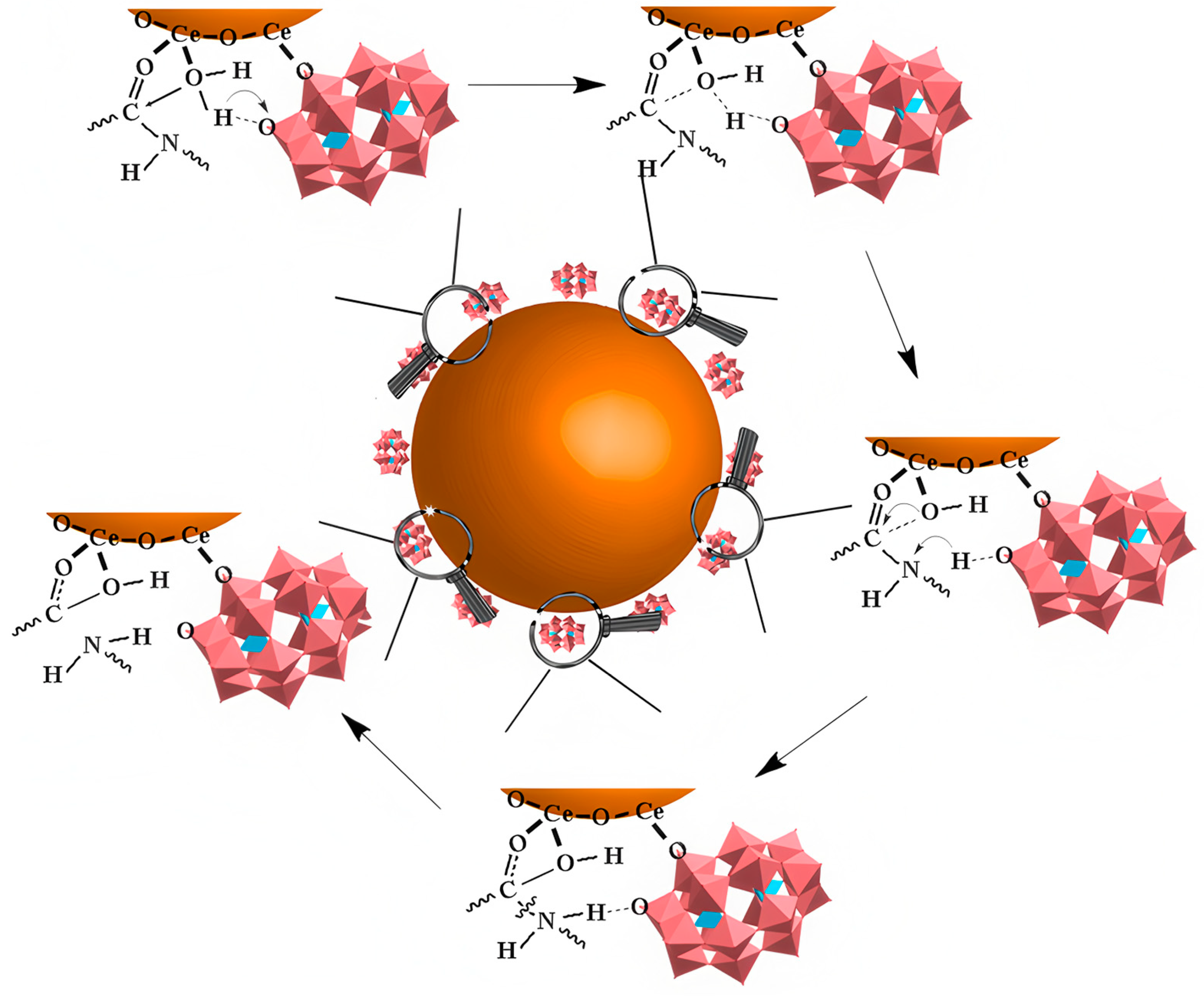
| 21 | Aβ | CeONP@POMs * | POMD catalyzes Aβ peptide bond cleavage, electrostatic interactions inhibit/disaggregate Aβ aggregation, Ce3+/Ce4+ redox activity cooperatively scavenges ROS. | Intracellular ROS reduction rate: 68% (Aβ40-induced PC12 cells); BBB penetration efficiency: 4.4 ± 0.46% (in vitro), ~0.54% (in vivo). | Aβ cleavage + Aβ aggregation inhibition/disaggregation + ROS scavenging | [60] |
| 22 | S100A9 | [N(CH3)4]6[Nb10O28], [N(CH3)4]7[TiNb9O28] | By electrostatic interactions with Lys-rich regions on the S100A9 surface, it induces local conformational changes that inhibit amyloid aggregation. | Nb10: S100A9 binding Kd: 2.86 ± 0.39 μM (intrinsic fluorescence), 1.17 ± 0.03 μM (ANS); TiNb9: S100A9 binding Kd: 2.48 ± 0.2 μM (intrinsic fluorescence), 0.45 ± 0.03 μM (ANS). | [61] | |
| 23 | AchE, BchE | Na10[H2W12O42] | Inhibition of AChE/BChE via non-competitive mechanism. | AChE IC50: 2.30 ± 0.44 μM; BChE IC50: 1.56 ± 0.46 μM. | [41] | |
| 24 | AchE, BchE | Na16[(O3POPO3)4W12O36] | Inhibition of AChE/BChE via electrostatic binding-altered active site. | AChE IC50: 3.51 ± 1.84 μM; BChE IC50: 0.18 ± 0.05 μM. | [41] | |
| 25 | AchE, BchE | Na16[(O3PCH2PO3)4W12O36] | Inhibition of AChE/BChE via non-competitive mechanism. | AChE IC50: 5.04 ± 1.06 μM; BChE IC50: 0.18 ± 0.05 μM. | [41] | |
| 26 | AchE, BchE | Na6[TeW6O24] | Inhibition of AChE/BChE via non-competitive mechanism. | AChE IC50: 0.31 ± 0.01 μM; BChE IC50: 0.46 ± 0.01 μM. | [41] | |
| 27 | AchE | K7[Ti2PW10O40], K6H[SiV3W9O40] | Inhibition of AChE activity via inducing AChE conformation perturbation. | K7[Ti2PW10O40]: AChE inhibition IC50: 1.04 × 10−6 mol/L; K6H[SiV3W9O40]: AChE inhibition IC50: 4.80 × 10−4 mol/L. | [62] | |
| 28 | AchE | H4[SiW12O40], H3[PW12O40] | Through electrostatic and hydrogen-bond interactions with the β-allosteric site (β-AS) of AChE, enzymatic activity is inhibited, preventing the α-helix-to-β-sheet transition of the AChE586–599 region. | WSiA: AChE inhibition IC50: 72.3 ± 0.2 nM; logP: −0.47; Hill coefficient: 0.93 ± 0.09; Cytostasis: 2.62%~11.24% (1 × 10−6~1 × 10−4 M) WPA: AChE inhibition IC50: 1230.0 ± 10.0 nM; logP: −0.29; Hill coefficient: 1.23 ± 0.17; Cytostasis: 7.87%~11.61% (1 × 10−6~1 × 10−6 M) | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhang, L.; Lu, T.; Hua, Z.; Peng, S.; Du, H.; Zhai, X.; Cai, Z.; Hua, J.; Ma, X. Recent Advances in Polyoxometalates Targeting Proteins Associated with Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Applications. Int. J. Mol. Sci. 2026, 27, 1257. https://doi.org/10.3390/ijms27031257
Zhang L, Lu T, Hua Z, Peng S, Du H, Zhai X, Cai Z, Hua J, Ma X. Recent Advances in Polyoxometalates Targeting Proteins Associated with Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Applications. International Journal of Molecular Sciences. 2026; 27(3):1257. https://doi.org/10.3390/ijms27031257
Chicago/Turabian StyleZhang, Lijuan, Tinghao Lu, Ziqian Hua, Shiheng Peng, Haoming Du, Xiaoting Zhai, Zhiqiang Cai, Jiai Hua, and Xiang Ma. 2026. "Recent Advances in Polyoxometalates Targeting Proteins Associated with Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Applications" International Journal of Molecular Sciences 27, no. 3: 1257. https://doi.org/10.3390/ijms27031257
APA StyleZhang, L., Lu, T., Hua, Z., Peng, S., Du, H., Zhai, X., Cai, Z., Hua, J., & Ma, X. (2026). Recent Advances in Polyoxometalates Targeting Proteins Associated with Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Applications. International Journal of Molecular Sciences, 27(3), 1257. https://doi.org/10.3390/ijms27031257







