Bridging Osteoimmunology and Regenerative Therapy: The Role of MSCs and Extracellular Vesicles
Abstract
1. Introduction
2. Mesenchymal Stem Cells as Coordinators of Bone-Immune Communication
2.1. Phenotypic and Functional Features of MSCs in the Bone Marrow Niche
2.2. MSC Crosstalk with Innate Immune Cells
2.3. MSC Modulation of Adaptive Immunity
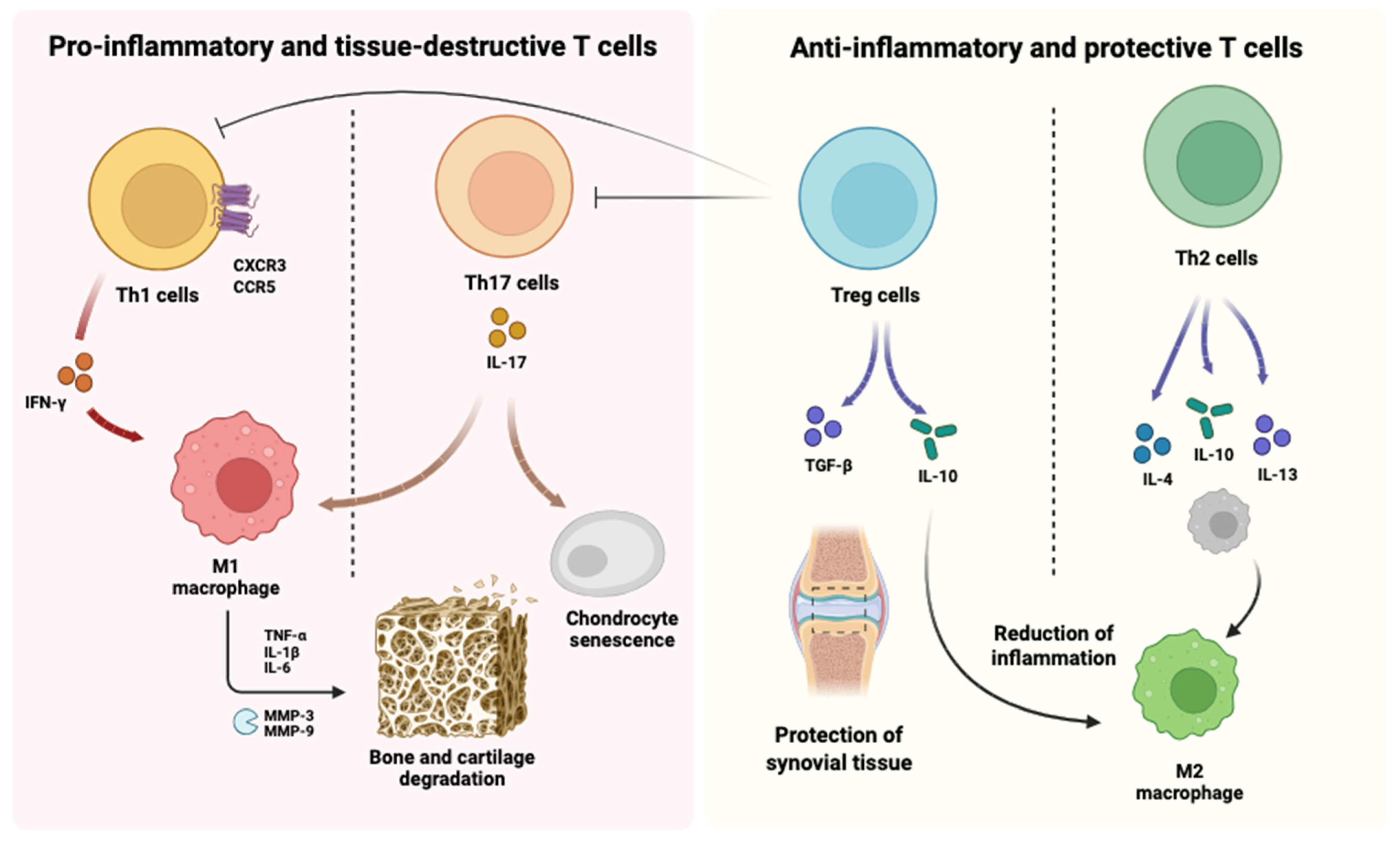
2.3.1. Regulation of T Cell Subsets
2.3.2. Interactions with B Cells
2.4. MSCs as Paracrine Regulators of Bone Remodelling
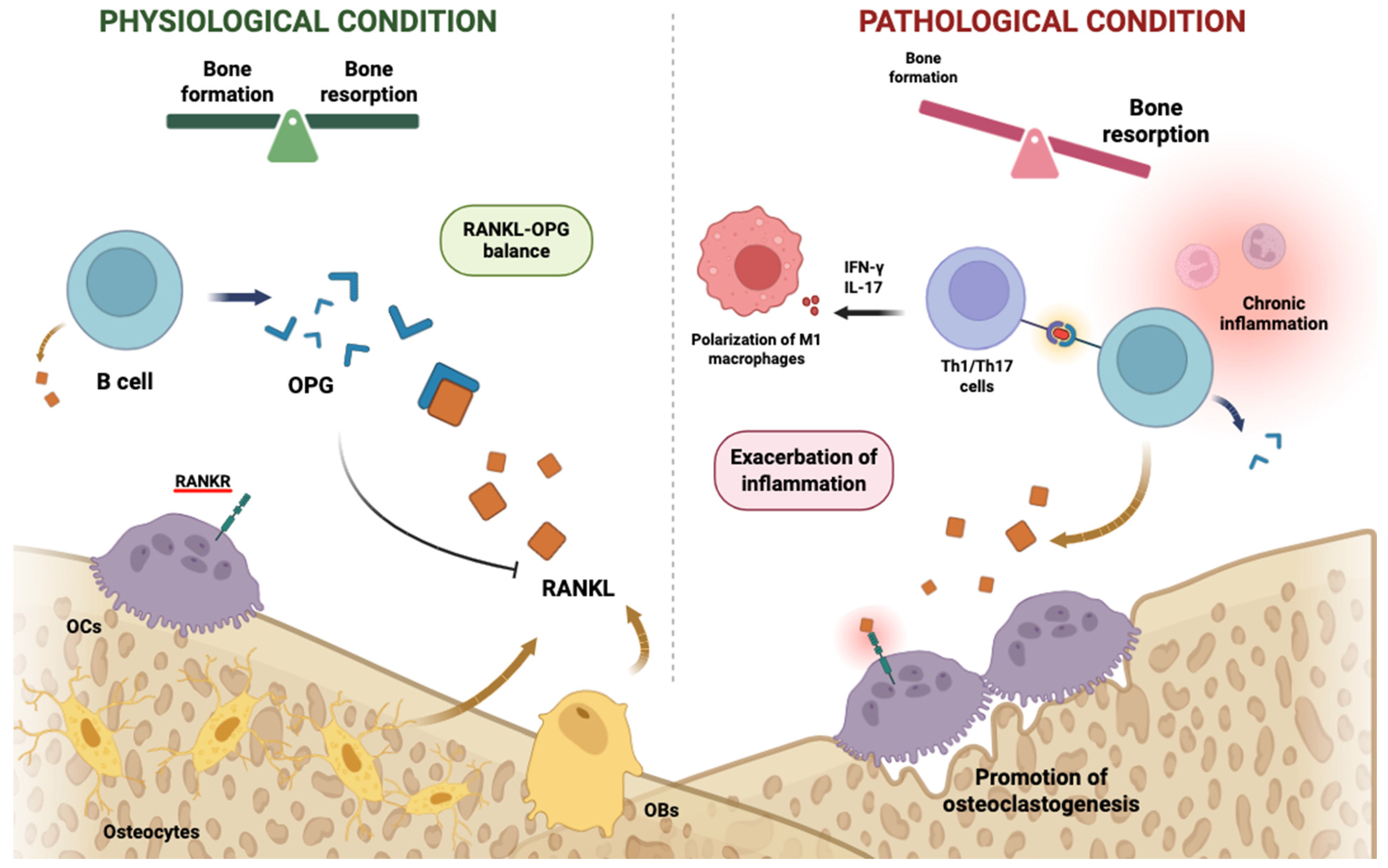
2.5. Implications for Osteoimmune Pathophysiology
3. Secretome and Extracellular Vesicles as Mediators of MSC-Driven Osteoimmune Regulation
3.1. Soluble Components of the MSC Secretome in Immune and Skeletal Regulation
3.1.1. Anti-Inflammatory Cytokines and Immunoregulatory Factors
3.1.2. Pro-Regenerative Growth Factors and Matrix Remodelling Enzymes
3.2. Modulation of Immune Cell Phenotypes by the MSC Secretome
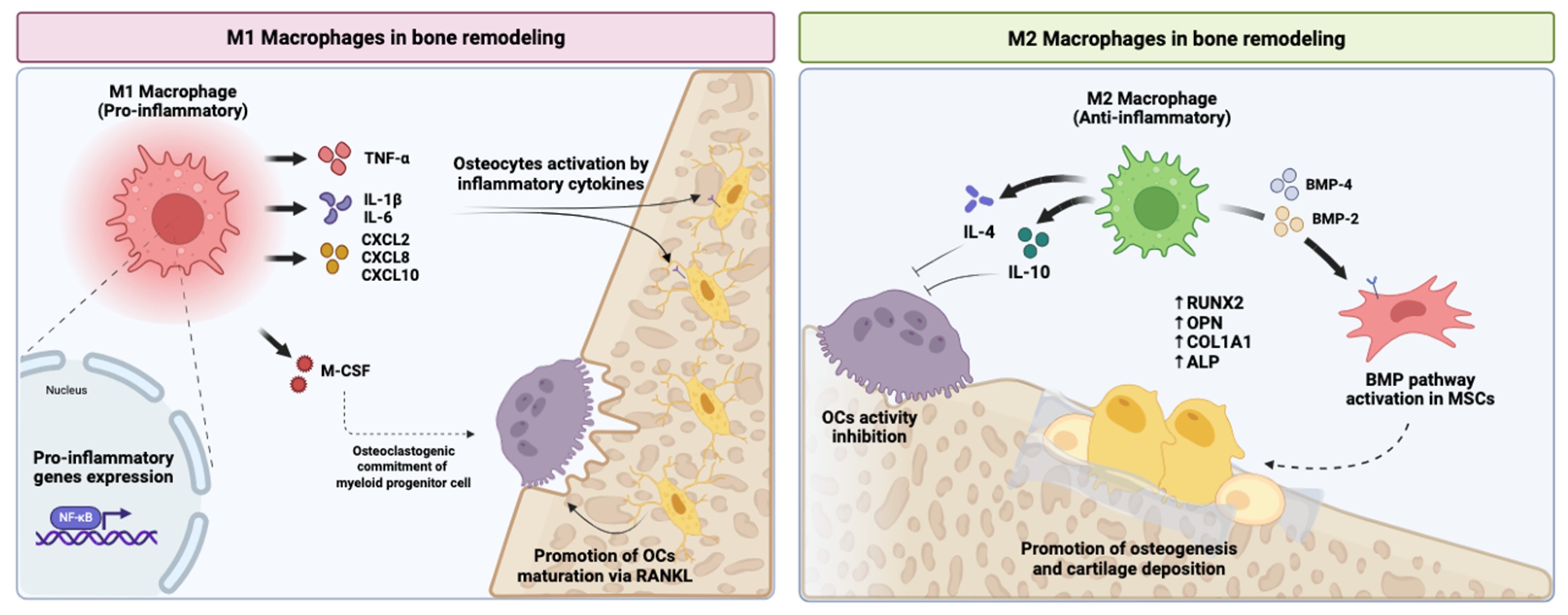
3.2.1. Macrophage Reprogramming and Inflammation Resolution
3.2.2. Regulation of T Cell and B Cell Responses
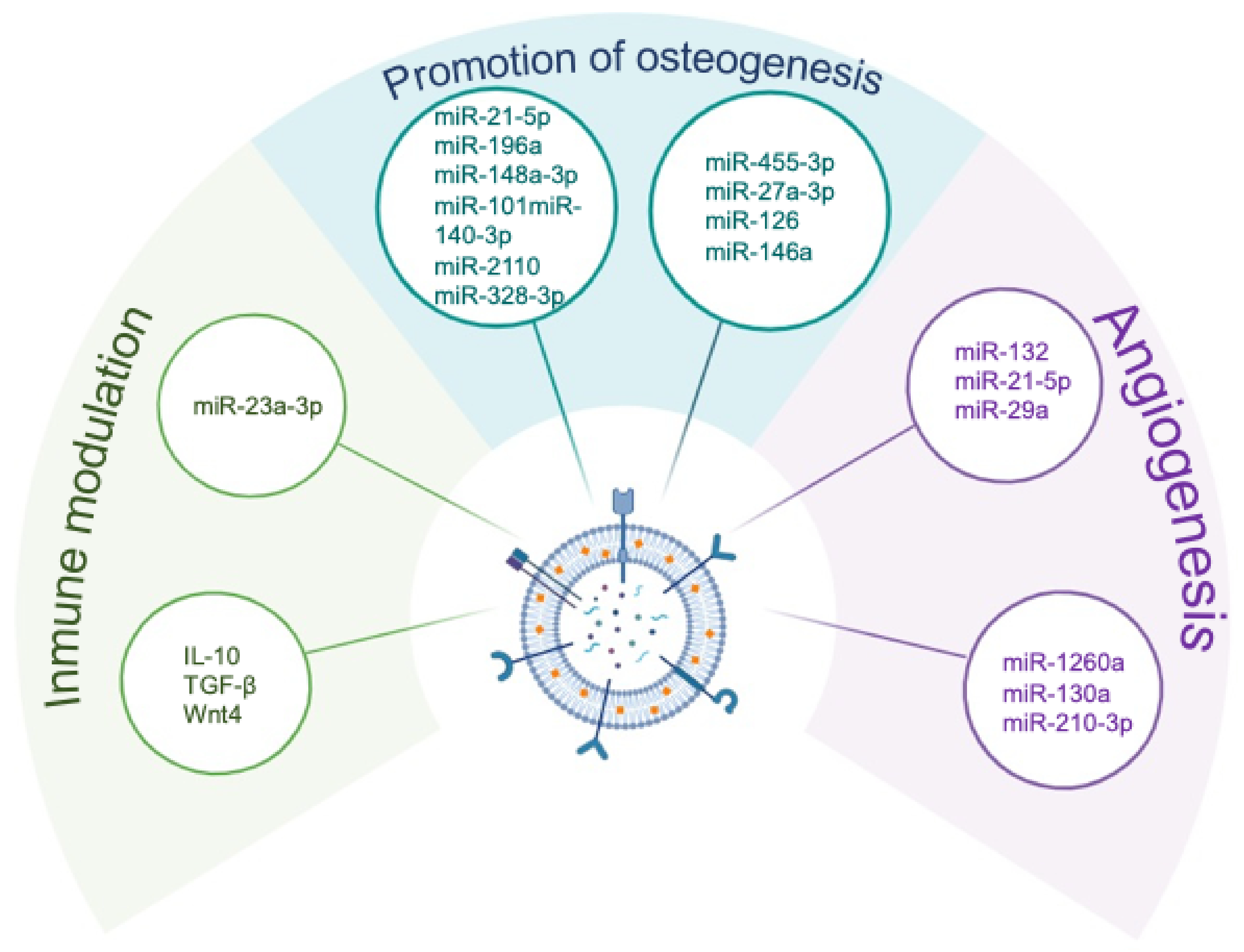
3.3. Extracellular Vesicles: Nanoscale Mediators of Osteoimmune Crosstalk
3.3.1. Biogenesis and Functional Repertoire of MSC-Derived EVs
3.3.2. Osteogenic Effects Mediated by EV Cargo
3.3.3. Immune Modulation Through EV-Mediated Signalling
3.4. Angiogenic and Vascular-Regenerative Roles of MSC-Derived EVs
3.5. Context-Dependent Effects and Potential Limitations of MSC-Derived Secretome and Extracellular Vesicles
4. Translational Development of the MSC Secretome and Extracellular Vesicles for Bone Repair
4.1. Rationale for Advancing Cell-Free Regenerative Strategies
4.2. Strategies for Enhancing the Therapeutic Potency of MSC Secretome and EVs
4.2.1. Preconditioning and Environmental Modulation of MSCs
4.2.2. Genetic Engineering Approaches to Enhance EV Content
4.3. Biomaterial-Based Delivery Systems for the Secretome and EVs
4.3.1. Hydrogels and Structural Scaffolds
4.3.2. Targeted Delivery and Bone-Specific Homing
4.4. Manufacturing, Quality Control, and Regulatory Barriers
4.5. Mechanistic Gaps and Future Biological Opportunities
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| ALP | Alkaline phosphatase |
| BMP | Bone morphogenetic protein |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| EVs | Extracellular vesicles |
| GMP | Good Manufacturing Practice |
| HGF | Hepatocyte growth factor |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem/stromal cell |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer |
| OPG | Osteoprotegerin |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PTEN | Phosphatase and tensin homolog |
| RANK | Receptor activator of nuclear factor κB |
| RANKL | Receptor activator of nuclear factor κB ligand |
| RUNX2 | Runt-related transcription factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor beta |
| Th | T helper |
| TLR | Toll-like receptor |
| TNF-α | Tumour necrosis factor alpha |
| Treg | Regulatory T cell |
| VEGF | Vascular endothelial growth factor |
References
- Hu, K.; Shang, Z.; Yang, X.; Zhang, Y.; Cao, L. Macrophage Polarization and the Regulation of Bone Immunity in Bone Homeostasis. J. Inflamm. Res. 2023, 16, 3563–3580. [Google Scholar] [CrossRef]
- Okamoto, K. Crosstalk between bone and the immune system. J. Bone Miner. Metab. 2024, 42, 470–480, Correction in J. Bone Miner. Metab. 2024, 42, 481–482. https://doi.org/10.1007/s00774-024-01547-x. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Grassi, F.; Ryan, M.R.; Terauchi, M.; Page, K.; Yang, X.; Weitzmann, M.N.; Pacifici, R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Investig. 2007, 117, 122–132. [Google Scholar] [CrossRef]
- Camacho, V.; Matkins, V.R.; Patel, S.B.; Lever, J.M.; Yang, Z.; Ying, L.; Landuyt, A.E.; Dean, E.C.; George, J.F.; Yang, H.; et al. Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight 2020, 5, 135681. [Google Scholar] [CrossRef]
- Capobianco, C.A.; Hankenson, K.D.; Knights, A.J. Temporal dynamics of immune-stromal cell interactions in fracture healing. Front. Immunol. 2024, 15, 1352819. [Google Scholar] [CrossRef] [PubMed]
- Levescot, A.; Chang, M.H.; Schnell, J.; Nelson-Maney, N.; Yan, J.; Martínez-Bonet, M.; Grieshaber-Bouyer, R.; Lee, P.Y.; Wei, K.; Blaustein, R.B.; et al. IL-1β-driven osteoclastogenic Tregs accelerate bone erosion in arthritis. J. Clin. Investig. 2021, 131, e141008. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J.; Xie, Z. The balance between helper T 17 and regulatory T cells in osteoimmunology and relevant research progress on bone tissue engineering. Immun. Inflamm. Dis. 2024, 12, e70011. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.P. The roles and regulatory mechanisms of TGF-beta and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858, Correction in Stem Cells 2017, 35, 2103. https://doi.org/10.1002/stem.2626. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Boilard, E. Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59, 2037–2046. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, W.; Dai, L.; Chen, L. The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases. Curr. Issues Mol. Biol. 2024, 46, 9269–9285. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, W.; Yang, Q.; Yang, S.; Yang, Y.; Fan, L.; Zhang, Y.; Qi, B.; Shi, Z.; Wei, X.; et al. Non-Coding-RNA-Activated Core/Chitosan Shell Nanounits Coated with Polyetheretherketone for Promoting Bone Regeneration and Osseointegration via Osteoimmunology. ACS Appl. Mater. Interfaces 2023, 15, 12653–12668. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Muñoz, J.; Akhavan, N.S.; Mullins, A.P.; Arjmandi, B.H. Macrophage polarization and osteoporosis: A review. Nutrients 2020, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Li, X.; Xiao, S.; Li, F.; Fang, K.; Wen, J.; Gong, H. Max interacting protein 1 induces IL-17-producing T helper/regulatory T imbalance in osteoarthritis by upregulating tectonic family member 2. Tissue Cell 2022, 78, 101906. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Q.; Li, Q.; Wen, J.; Li, M.; Wu, Z.; Nie, L.; Huang, Z.; Wu, S.Y.; Du, J. Metallothionein-1 mitigates the advancement of osteoarthritis by regulating Th17/Treg balance. Cell. Immunol. 2024, 405–406, 104877. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, A.; Garcia-Sanchez, D.; Dotta, M.; Rodriguez-Rey, J.C.; Perez-Campo, F.M. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J. Stem Cells 2020, 12, 1529–1552. [Google Scholar] [CrossRef]
- Guo, X.; Xu, T.; Zheng, J.; Cui, X.; Li, M.; Wang, K.; Su, M.; Zhang, H.; Zheng, K.; Sun, C.; et al. Accumulation of synovial fluid CD19(+)CD24(hi)CD27(+) B cells was associated with bone destruction in rheumatoid arthritis. Sci. Rep. 2020, 10, 14386. [Google Scholar] [CrossRef]
- Hu, K.; Song, M.; Song, T.; Jia, X.; Song, Y. Osteoimmunology in Osteoarthritis: Unraveling the Interplay of Immunity, Inflammation, and Joint Degeneration. J. Inflamm. Res. 2025, 18, 4121–4142. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Atta, O.M.; Kishta, M.S.; Maboruk, M.; Shi, Z.; Yang, G. Lysozyme-enhanced cellulose nanofiber, chitosan, and graphene oxide multifunctional nanocomposite for potential burn wound healing applications. Int. J. Biol. Macromol. 2024, 280, 135668. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.A.; ElShebiney, S.A.; Mabrouk, M.; Kishta, M.S.; Galal, A.F.; Osama, L.; Beherei, H.H. Enhanced bone regeneration using mesenchymal stem cell-loaded 3D-printed alginate-calcium Titanate scaffolds: A Calvarial defect model study. Int. J. Biol. Macromol. 2025, 302, 140516. [Google Scholar] [CrossRef]
- Muñoz, M.; Robinson, K.; Shibli-Rahhal, A. Bone Health and Osteoporosis Prevention and Treatment. Clin. Obstet. Gynecol. 2020, 63, 770–787. [Google Scholar] [CrossRef]
- Salgado, A.J.; Gimble, J.M. Secretome of mesenchymal stem/stromal cells in regenerative medicine. Biochimie 2013, 95, 2195. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; Ahmed, H.H.; Mohamed, S.H.; Kotob, S.E.; Kishta, M.S. Nanotherapy: New Approach for Impeding Hepatic Cancer Microenvironment via Targeting Multiple Molecular Pathways. Asian Pac. J. Cancer Prev. 2022, 23, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabou, A.A.; Kamal, M.; Alharbi, H.Y.; Aljohani, M.S.; El-Atawy, M.A.; Kishta, M.S. Modulation of PI3K/AKT signaling and DFT modeling via selected pharmaceutical compounds attenuates carrageenan-induced inflammation and oxidative stress in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 7583–7603. [Google Scholar] [CrossRef]
- Aglan, H.A.; Ahmed, H.H.; Beherei, H.H.; Abdel-Hady, B.M.; Ekram, B.; Kishta, M.S. Generation of cardiomyocytes from stem cells cultured on nanofibrous scaffold: Experimental approach for attenuation of myocardial infarction. Tissue Cell 2024, 89, 102461. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.L.; Ramos-Rodriguez, D.H.; Mesfin, M.; Leach, J.K. Hydrogel degradation promotes angiogenic and regenerative potential of cell spheroids for wound healing. Mater. Today Bio 2023, 22, 100769. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Mao, F.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, M.; Yan, Y.; Yang, T.; Zhang, J.; Zhu, W.; et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappaB and signal transducer and activator of transcription 3 pathways. Exp. Biol. Med. 2014, 239, 366–375. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Ghannam, S.; Pene, J.; Moquet-Torcy, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312, Correction in J. Immunol. 2013, 191, 5777. https://doi.org/10.4049/jimmunol.1390061. [Google Scholar] [CrossRef]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Rafei, M.; Campeau, P.M.; Aguilar-Mahecha, A.; Buchanan, M.; Williams, P.; Birman, E.; Yuan, S.; Young, Y.K.; Boivin, M.N.; Forner, K.; et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 2009, 182, 5994–6002. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Wu, P.; Xiao, Y.; Duan, N.; Quan, J.; Du, W. microRNA-148a-3p in extracellular vesicles derived from bone marrow mesenchymal stem cells suppresses SMURF1 to prevent osteonecrosis of femoral head. J. Cell. Mol. Med. 2020, 24, 11512–11523. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xiong, Y.; Sun, Y.; Zeng, R.; Xue, H.; Hu, Y.; Chen, L.; Liu, G.; Panayi, A.C.; Zhou, W.; et al. Circulating MiRNA-21-enriched extracellular vesicles promote bone remodeling in traumatic brain injury patients. Exp. Mol. Med. 2023, 55, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, K.; Zhou, H.; Li, G.; Xu, L.; Hu, Z.; Cao, X.; Shi, F.; Zhang, S. Circulating Exosomes from Mice with LPS-Induced Bone Loss Inhibit Osteoblast Differentiation. Calcif. Tissue Int. 2022, 111, 185–195. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ma, Y.; Du, W.; Feng, K.; Wang, S. miR-101-loaded exosomes secreted by bone marrow mesenchymal stem cells requires the FBXW7/HIF1alpha/FOXP3 axis, facilitating osteogenic differentiation. J. Cell. Physiol. 2021, 236, 4258–4272. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, M.; Jia, Z.; Chen, X.; Bu, N. MicroRNA-455-3p promotes osteoblast differentiation via targeting HDAC2. Injury 2022, 53, 3636–3641. [Google Scholar] [CrossRef]
- Ren, L.R.; Yao, R.B.; Wang, S.Y.; Gong, X.D.; Xu, J.T.; Yang, K.S. MiR-27a-3p promotes the osteogenic differentiation by activating CRY2/ERK1/2 axis. Mol. Med. 2021, 27, 43. [Google Scholar] [CrossRef]
- Hu, H.; Hu, X.; Li, L.; Fang, Y.; Yang, Y.; Gu, J.; Xu, J.; Chu, L. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p. Biomolecules 2022, 12, 883. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell. Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Mao, F.; Wu, Y.; Tang, X.; Kang, J.; Zhang, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed. Res. Int. 2017, 2017, 5356760. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Feng, C.; Chang, J.; Fu, R.; Wu, T.; Yu, F.; Wang, X.; Xia, L.; Wu, C.; et al. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials 2019, 192, 523–536. [Google Scholar] [CrossRef]
- Lu, G.D.; Cheng, P.; Liu, T.; Wang, Z. BMSC-Derived Exosomal miR-29a Promotes Angiogenesis and Osteogenesis. Front. Cell. Dev. Biol. 2020, 8, 608521. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.A.; Layrolle, P.; Mooney, D.J. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909125. [Google Scholar] [CrossRef]
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived sEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal miR-21. Front. Bioeng. Biotechnol. 2021, 9, 829136. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Garcia-Sanchez, D.; Gonzalez-Gonzalez, A.; Alvarez-Iglesias, I.; Dujo-Gutierrez, M.D.; Bolado-Carrancio, A.; Certo, M.; Perez-Nunez, M.I.; Riancho, J.A.; Rodriguez-Rey, J.C.; Delgado-Calle, J.; et al. Engineering a Pro-Osteogenic Secretome through the Transient Silencing of the Gene Encoding Secreted Frizzled Related Protein 1. Int. J. Mol. Sci. 2023, 24, 12399. [Google Scholar] [CrossRef]
- Khatab, S.; van Osch, G.J.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.; Bernsen, M.R.; van Buul, G.M. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur. Cells Mater. 2018, 36, 218–230. [Google Scholar] [CrossRef]
- Muzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, F.; Li, L.; Zhou, L.; Zhou, T.; Xu, Y.; Lin, K.; Fang, B.; Xia, L. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: Release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater. 2021, 119, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Jin, Q.; Ming-Yu, Y.; Yang, H.; Xu, T.; You-Xing, S.; Xu-Ting, B.; Wan, C.; Yun-Jiao, W.; Huan, W.; et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRalpha(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials 2021, 279, 121242. [Google Scholar] [CrossRef]
- Hung, M.E.; Leonard, J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell Vesicles 2016, 5, 31027. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.; Ryu, S.W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef]
- Ilahibaks, N.F.; Ardisasmita, A.I.; Xie, S.; Gunnarsson, A.; Brealey, J.; Vader, P.; de Jong, O.G.; de Jager, S.; Dekker, N.; Peacock, B.; et al. TOP-EVs: Technology of Protein delivery through Extracellular Vesicles is a versatile platform for intracellular protein delivery. J. Control Release 2023, 355, 579–592. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D Printing of Microenvironment-Specific Bioinspired and Exosome-Reinforced Hydrogel Scaffolds for Efficient Cartilage and Subchondral Bone Regeneration. Adv. Sci. 2023, 10, e2303650. [Google Scholar] [CrossRef]
- Villani, C.; Murugan, P.; George, A. Exosome-Laden Hydrogels as Promising Carriers for Oral and Bone Tissue Engineering: Insight into Cell-Free Drug Delivery. Int. J. Mol. Sci. 2024, 25, 11092. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater. 2022, 10, 207–221. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Cai, L.; Liu, T.; Wang, X.; Zhang, Y.; Zhou, Z.; Li, T.; Liu, M.; Lai, R.; et al. Bone-Targeted Extracellular Vesicles from Mesenchymal Stem Cells for Osteoporosis Therapy. Int. J. Nanomed. 2020, 15, 7967–7977. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Ma, H.W.; Xiang, G.H.; He, G.L.; Cai, H.C.; Dai, Z.H.; Chen, Y.L.; Lin, Y.; Xu, H.Z.; Ni, W.F.; et al. Bone-targeting delivery of platelet lysate exosomes ameliorates glucocorticoid-induced osteoporosis by enhancing bone-vessel coupling. J. Nanobiotechnol. 2022, 20, 220. [Google Scholar] [CrossRef]
- Beider, K.; Nagler, A.; Wald, O.; Franitza, S.; Dagan-Berger, M.; Wald, H.; Giladi, H.; Brocke, S.; Hanna, J.; Mandelboim, O.; et al. Involvement of CXCR4 and IL-2 in the homing and retention of human NK and NK T cells to the bone marrow and spleen of NOD/SCID mice. Blood 2003, 102, 1951–1958. [Google Scholar] [CrossRef]
- Lapidot, T.; Kollet, O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 2002, 16, 1992–2003. [Google Scholar] [CrossRef]
- Chewchuk, S.; Soucy, N.; Wan, F.; Harden, J.; Godin, M. pH controlled release of extracellular vesicles from a hydrogel scaffold for therapeutic applications. Biomed. Mater. 2025, 20, 065006. [Google Scholar] [CrossRef]
- Safwan, M.; Bourgleh, M.S.; Aldoush, M.; Haider, K.H. Tissue-source effect on mesenchymal stem cells as living biodrugs for heart failure: Systematic review and meta-analysis. World J. Cardiol. 2024, 16, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C. Pathophysiology of bone remodelling cycle: Role of immune system and lipids. Biochem. Pharmacol. 2025, 235, 116844. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gao, L.; Chang, R.; Zhai, L.; Zhao, Y. Crosstalk between macrophages and immunometabolism and their potential roles in tissue repair and regeneration. Heliyon 2024, 10, e38018. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Zhu, G.; Ma, Q.; Huang, S.; Guo, G.; Zhu, F. Application progress of single-cell sequencing technology in mesenchymal stem cells research. Front. Cell Dev. Biol. 2023, 11, 1336482. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Álvarez-Iglesias, I.; Colombo, A.; Gil-de-Gómez, L.; García-Sánchez, D.; González-González, A.; Pérez-Campo, F.M. Bridging Osteoimmunology and Regenerative Therapy: The Role of MSCs and Extracellular Vesicles. Int. J. Mol. Sci. 2026, 27, 1155. https://doi.org/10.3390/ijms27031155
Álvarez-Iglesias I, Colombo A, Gil-de-Gómez L, García-Sánchez D, González-González A, Pérez-Campo FM. Bridging Osteoimmunology and Regenerative Therapy: The Role of MSCs and Extracellular Vesicles. International Journal of Molecular Sciences. 2026; 27(3):1155. https://doi.org/10.3390/ijms27031155
Chicago/Turabian StyleÁlvarez-Iglesias, Itziar, Alice Colombo, Luis Gil-de-Gómez, Daniel García-Sánchez, Alberto González-González, and Flor M. Pérez-Campo. 2026. "Bridging Osteoimmunology and Regenerative Therapy: The Role of MSCs and Extracellular Vesicles" International Journal of Molecular Sciences 27, no. 3: 1155. https://doi.org/10.3390/ijms27031155
APA StyleÁlvarez-Iglesias, I., Colombo, A., Gil-de-Gómez, L., García-Sánchez, D., González-González, A., & Pérez-Campo, F. M. (2026). Bridging Osteoimmunology and Regenerative Therapy: The Role of MSCs and Extracellular Vesicles. International Journal of Molecular Sciences, 27(3), 1155. https://doi.org/10.3390/ijms27031155






