Venom-Derived Proteins from Lonomia obliqua Modulate Cytoskeletal Regulators and Inflammatory Responses in Human Chondrocytes
Abstract
1. Introduction
2. Results
2.1. Recombinant Proteins Preparation
2.2. LOCBE and Recombinant Proteins Are Not Toxic to Chondrocytes, and LOCBE Attenuates IL-1β–Induced Cytotoxic Effects
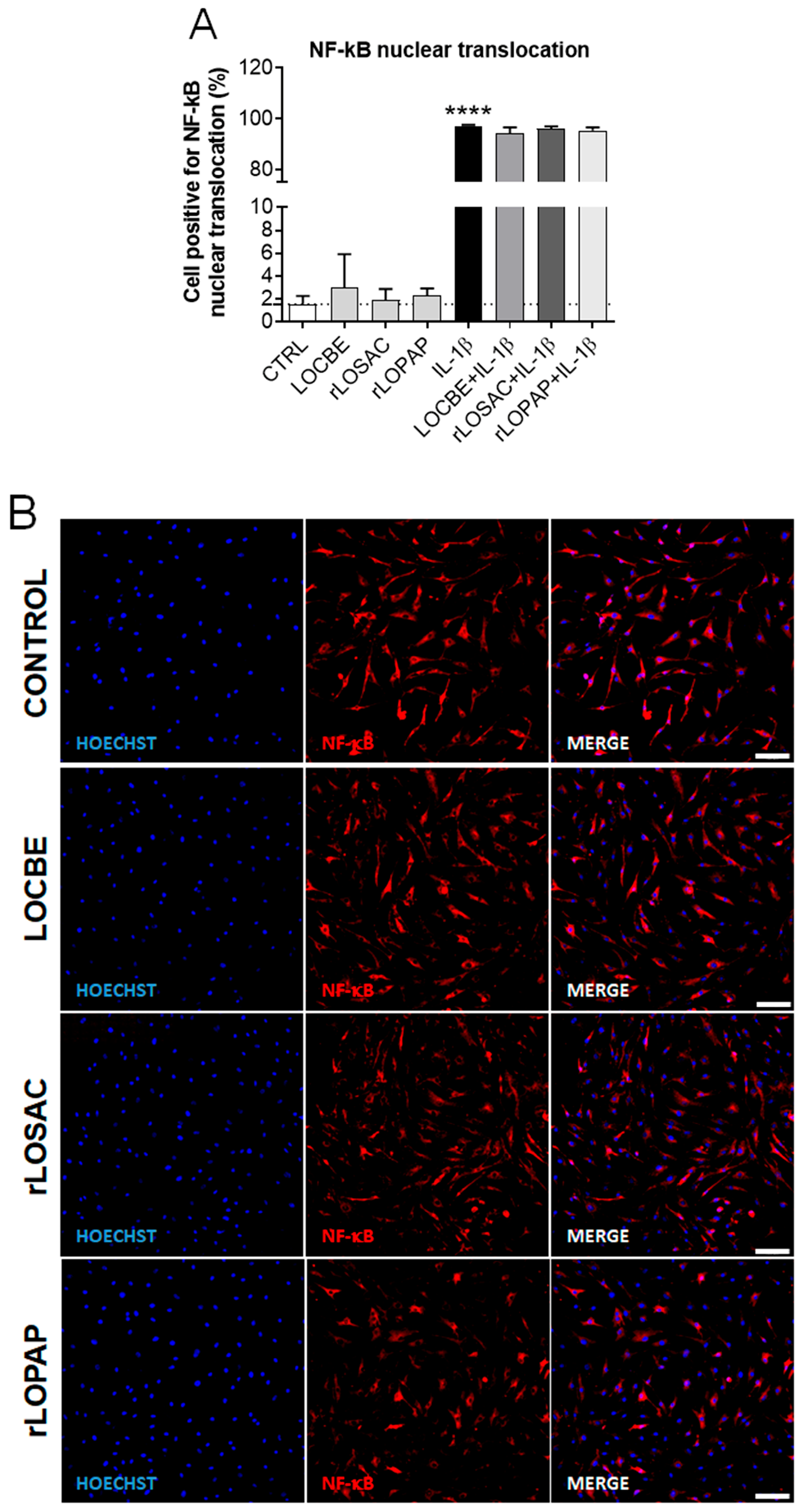
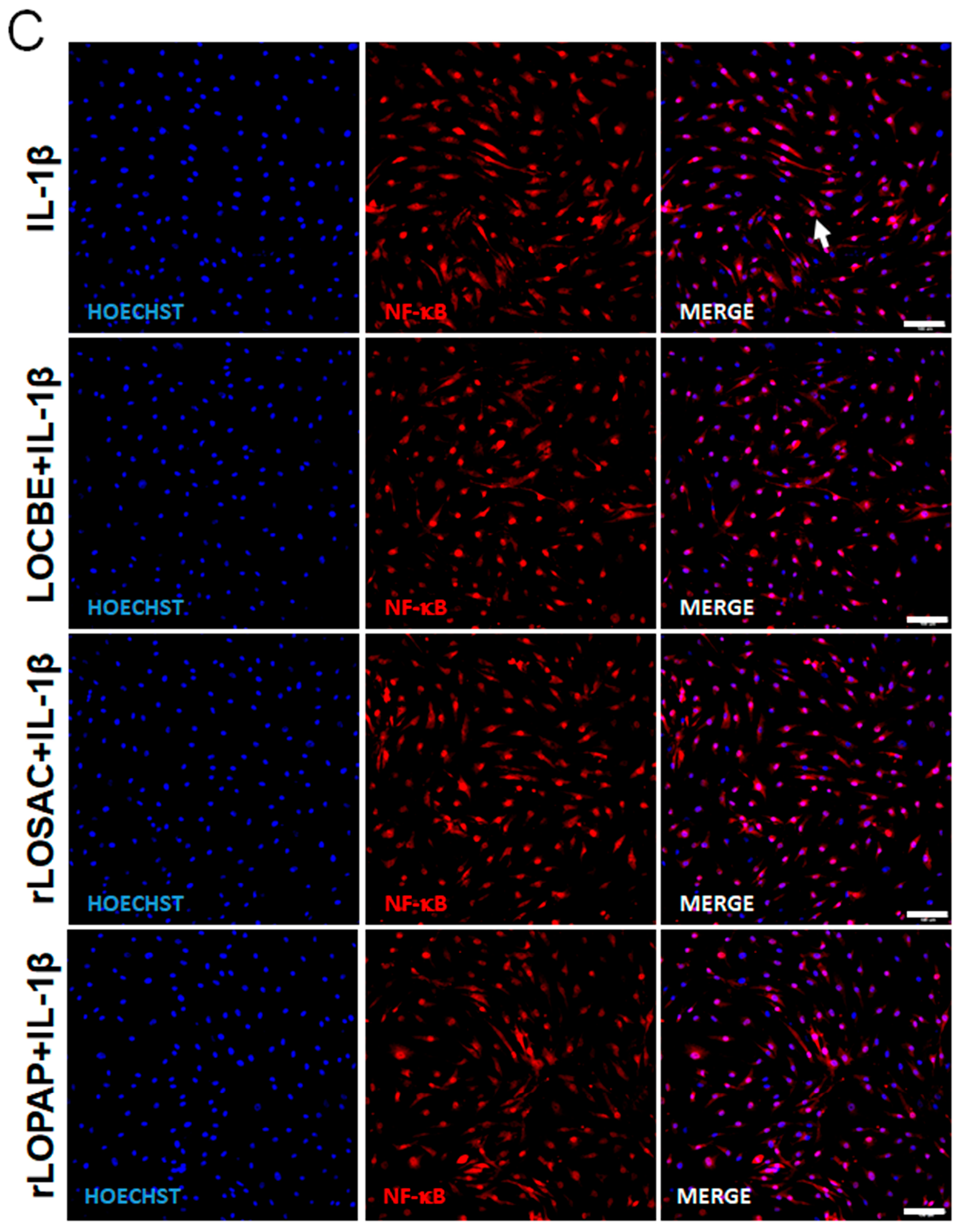
2.3. LOCBE, rLOPAP, or rLOSAC Do Not Affect NF-κB Translocation but Induce the Release of Inflammatory Cytokines
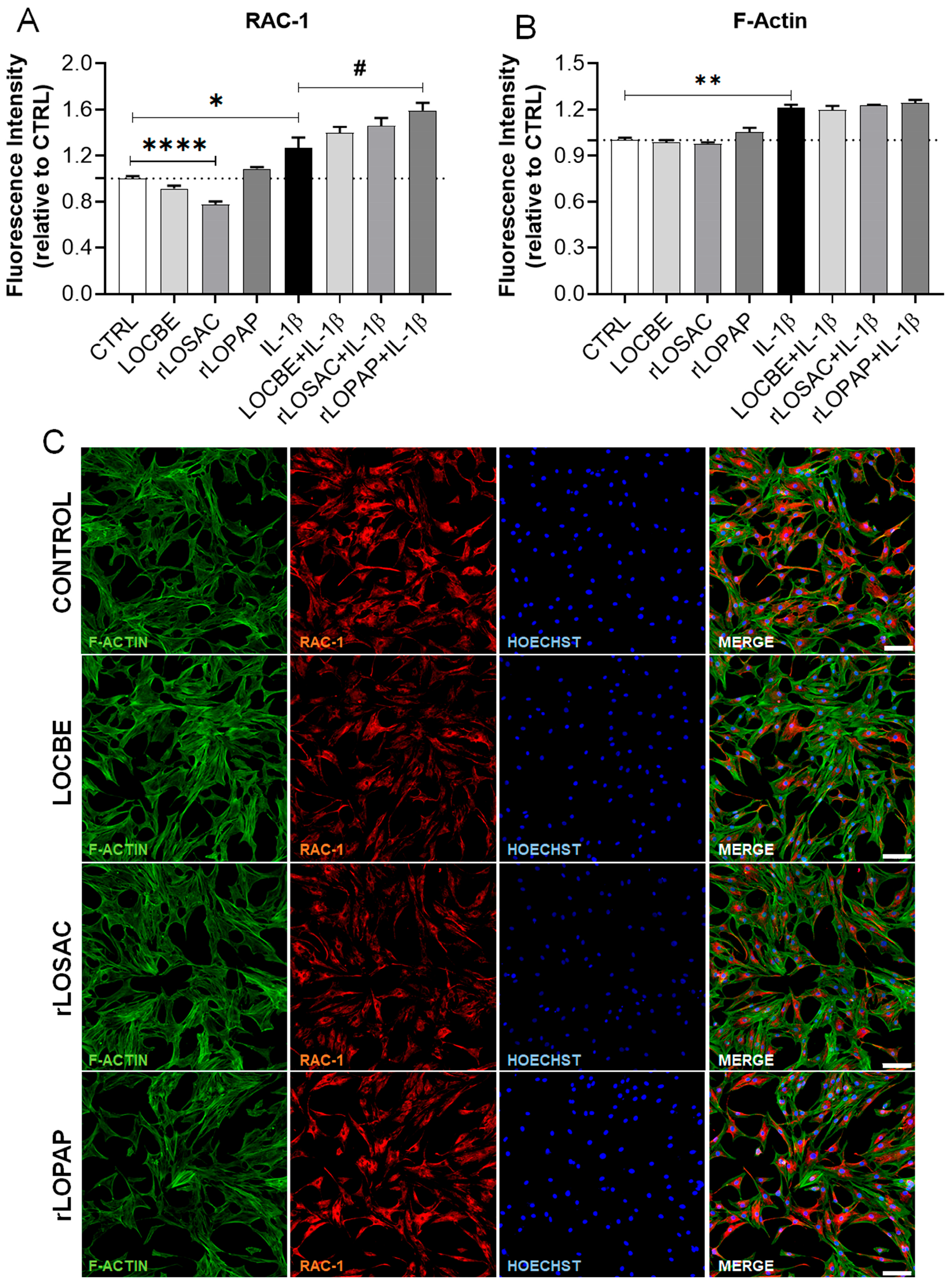
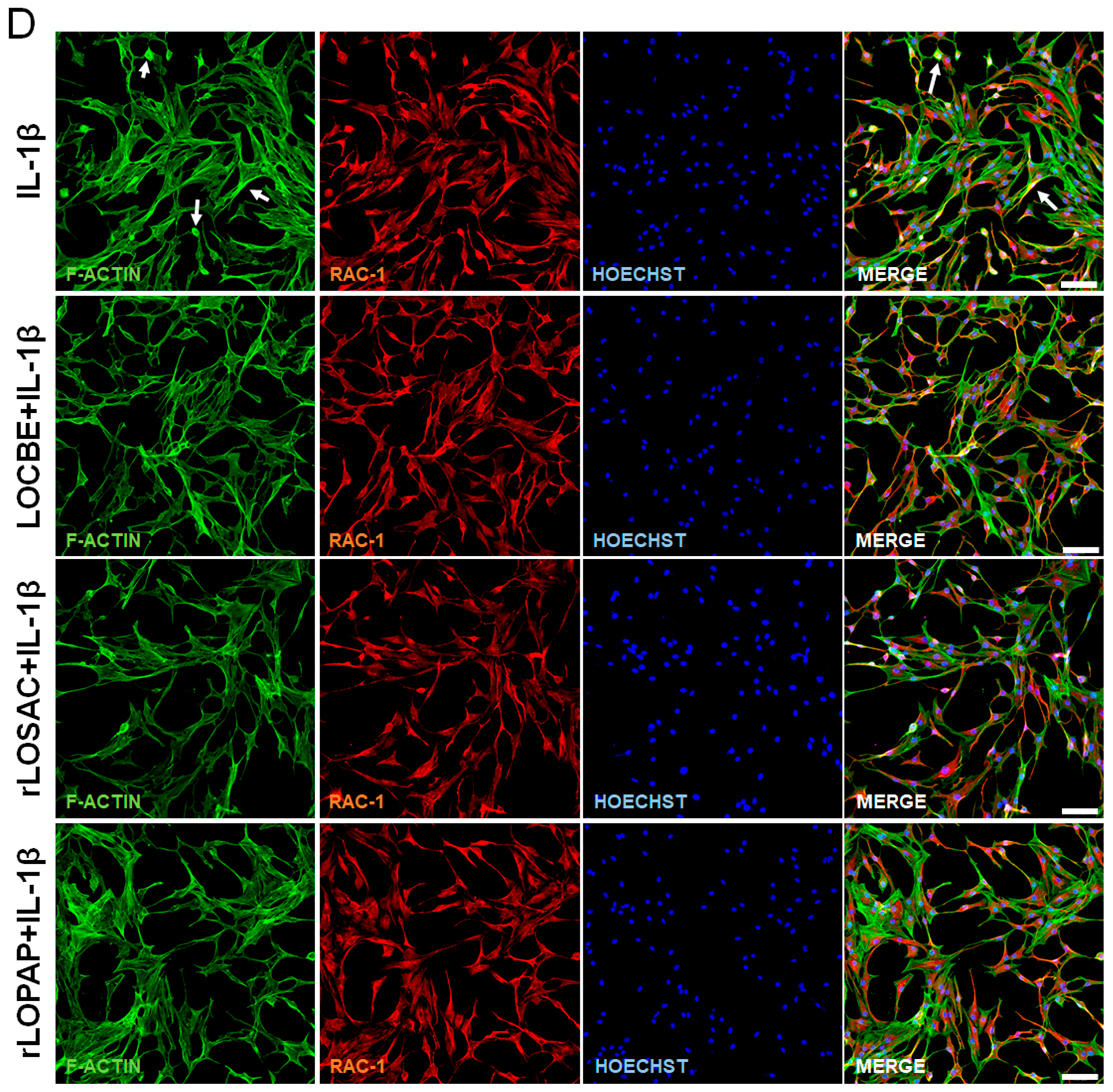
2.4. Morphological Changes Suggest Alterations in Cytoskeletal Proteins and Rac-1 Protein
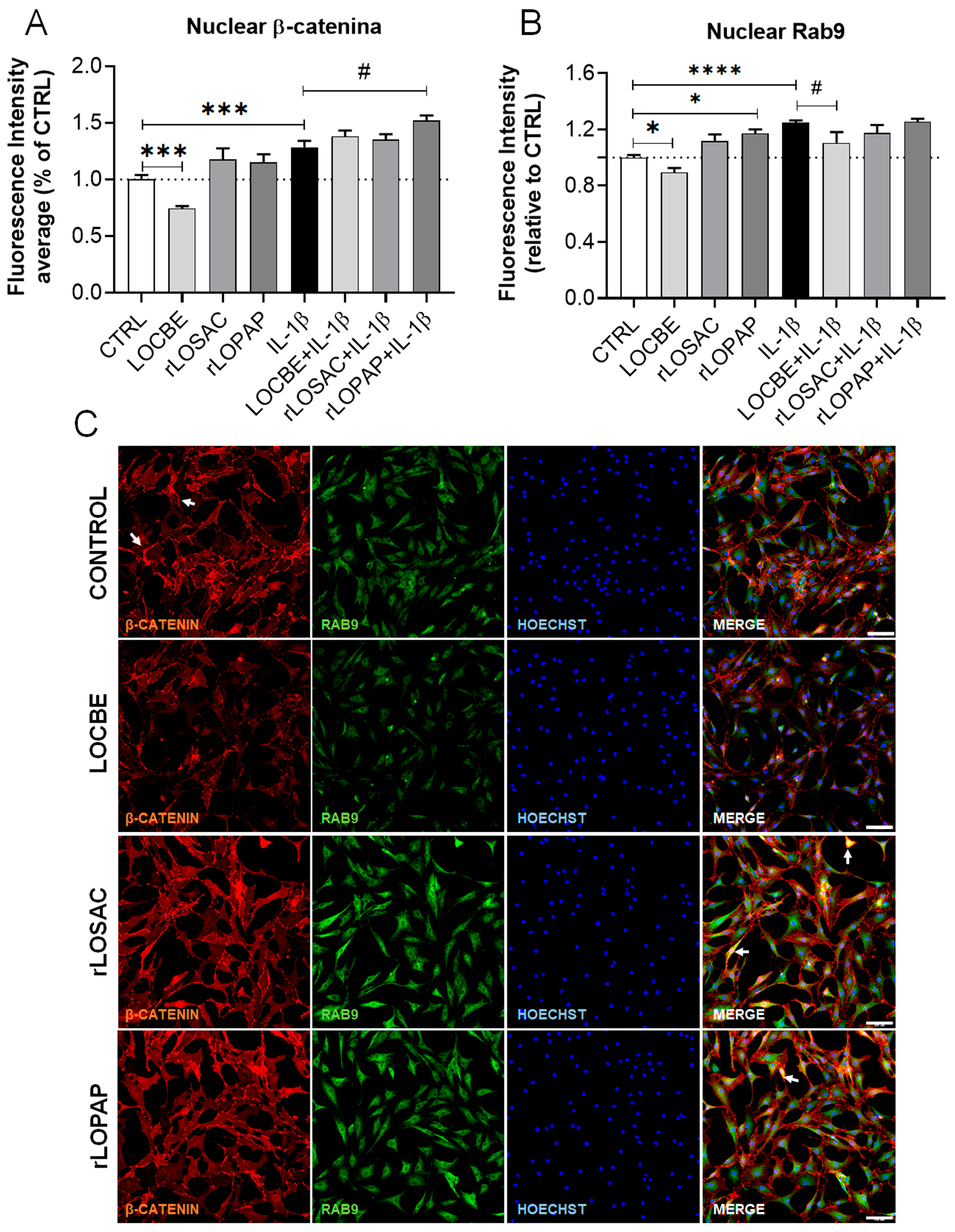
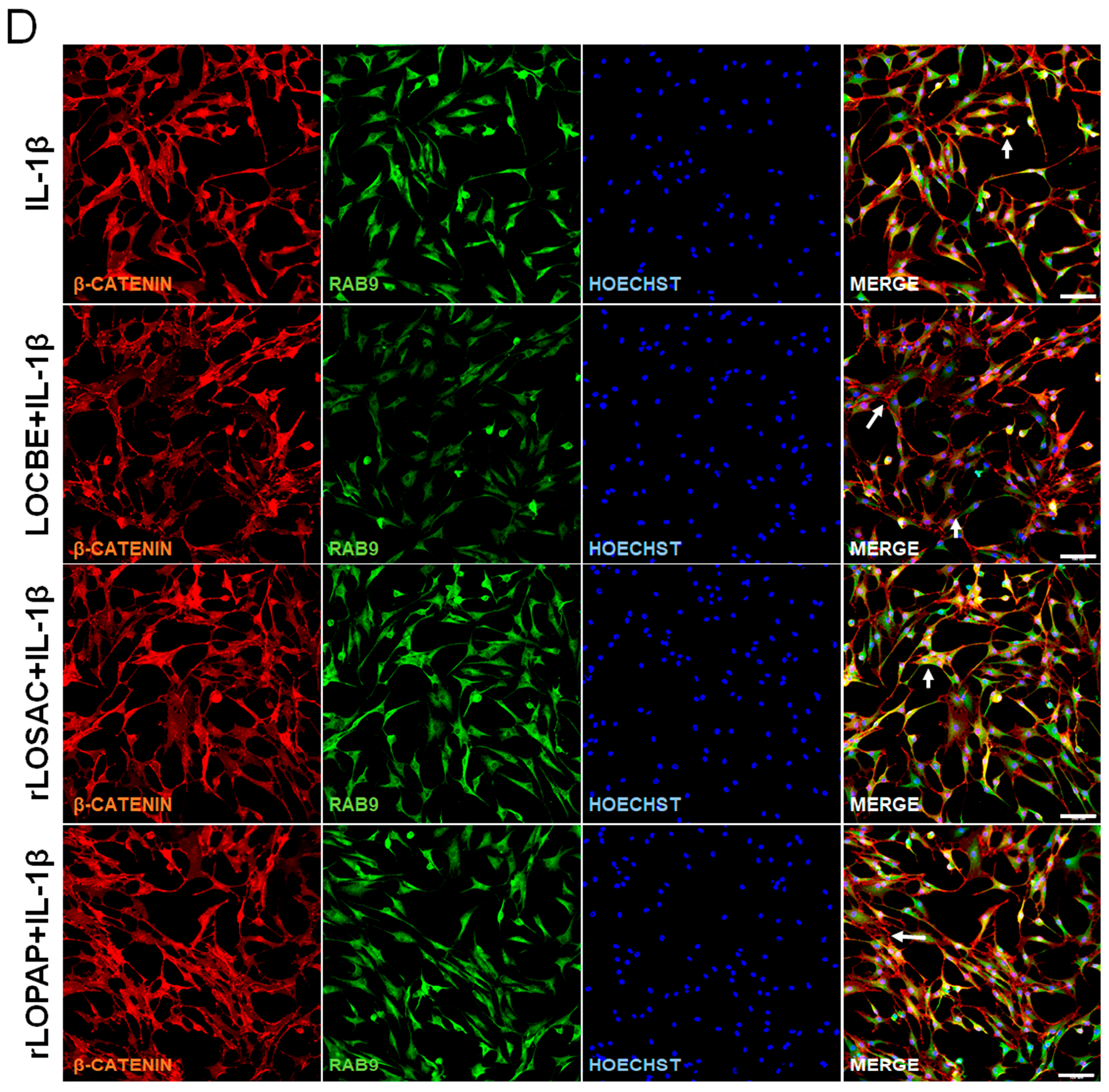
2.5. Effect of LOCBE and Recombinant Proteins on the Expression of RhoA, β-Catenin and Rab-9
3. Discussion
4. Materials and Methods
4.1. Venom and Proteins Preparation
4.2. Cell Culture
4.3. Chondrocyte Viability Assay
4.4. Cytokine Release Analysis
4.5. Immunofluorescence, Acquisition, and Image-Based Analysis
4.5.1. Immunofluorescence Staining Protocol
4.5.2. Image Acquisition, Analysis, and Quantification
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Varela-Eirin, M.; Loureiro, J.; Fonseca, E.; Corrochano, S.; Caeiro, J.R.; Collado, M.; Mayan, M.D. Cartilage regeneration and ageing: Targeting cellular plasticity in osteoarthritis. Ageing Res. Rev. 2018, 42, 56–71. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Chen, N.; Xu, S.; Zhang, Y.; Wang, F. Animal protein toxins: Origins and therapeutic applications. Biophys. Rep. 2018, 4, 233–242. [Google Scholar] [CrossRef]
- Utkin, Y. Animal Venoms and Their Components: Molecular Mechanisms of Action. Toxins 2021, 13, 415. [Google Scholar] [CrossRef]
- Alvarez-Flores, M.P.; Correia Batista, I.F.; Villas Boas, I.M.; Bufalo, M.C.; de Souza, J.G.; Oliveira, D.S.; Bonfá, G.; Fernandes, C.M.; Marques Porto, R.; Lichtenstein, F.; et al. Snake and arthropod venoms: Search for inflammatory activity in human cells involved in joint diseases. Toxicon 2024, 238, 107568. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.L.; Pohl, P.C.; Villas-Boas, I.M.; Pidde, G.; Tambourgi, D.V. Investigating the impact of Premolis semirufa caterpillar bristle toxins on human chondrocyte activation and inflammation. PLoS Neglected Trop. Dis. 2025, 19, e0012816. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flores, M.P.; Gomes, R.N.; Trevisan-Silva, D.; Oliveira, D.S.; Batista, I.F.C.; Buri, M.V.; Alvarez, A.M.; DeOcesano-Pereira, C.; de Souza, M.M.; Chudzinski-Tavassi, A.M. Lonomia obliqua Envenoming and Innovative Research. Toxins 2021, 13, 832. [Google Scholar] [CrossRef]
- Alvarez, A.M.; Alvarez-Flores, M.P.; DeOcesano-Pereira, C.; Goldfeder, M.B.; Chudzinski-Tavassi, A.M.; Moreira, V.; Teixeira, C. Losac and Lopap Recombinant Proteins from Lonomia obliqua Bristles Positively Modulate the Myoblast Proliferation Process. Front. Mol. Biosci. 2022, 9, 904737. [Google Scholar] [CrossRef]
- Bernardi, L.; Pinto, A.F.M.; Mendes, E.; Yates, J.R., 3rd; Lamers, M.L. Lonomia obliqua bristle extract modulates Rac-1 activation, membrane dynamics and cell adhesion properties. Toxicon 2019, 162, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Conboy, J.P.; Istúriz Petitjean, I.; van der Net, A.; Koenderink, G.H. How cytoskeletal crosstalk makes cells move: Bridging cell-free and cell studies. Biophys. Rev. 2024, 5, 021307. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Ladwein, M.; Dimchev, G.A.; Hein, A.; Schwenkmezger, L.; Arens, S.; Ladwein, K.I.; Margit Holleboom, J.; Schur, F.; Small, J.V.; et al. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J. Cell Sci. 2013, 126, 4572–4588. [Google Scholar] [CrossRef] [PubMed]
- Schaks, M.; Giannone, G.; Rottner, K. Actin dynamics in cell migration. Essays Biochem. 2019, 63, 483–495. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, P.; Liu, H.; Chen, P.; Wu, Y.; Wang, Y.; Sun, H.; Zhang, X.; Xia, Q.; Heng, B.C.; et al. Inhibition of Rac-1 activity by controlled release of NSC23766 from chitosan microspheres effectively ameliorates osteoarthritis development in vivo. Ann. Rheum. Dis. 2015, 74, 285–293. [Google Scholar] [CrossRef]
- Deng, Z.; Jia, Y.; Liu, H.; He, M.; Yang, Y.; Xiao, W.; Li, Y. RhoA/ROCK pathway: Implication in osteoarthritis and therapeutic targets. Am. J. Transl. Res. 2019, 11, 5324–5331. [Google Scholar]
- Woods, A.; Wang, G.; Beier, F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J. Cell. Physiol. 2007, 213, 1–8. [Google Scholar] [CrossRef]
- Long, D.L.; Willey, J.S.; Loesse, R.F. Rac-1 Is Required for Matrix Metalloproteinase 13 Production by Chondrocytes in Response to Fibronectin Fragments. Arthritis Rheum. 2013, 65, 1561–1568. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, Z.M.; Zhu, D.C.; Guo, Q.; Xu, J.J.; Lin, J.H.; Chen, Z.X.; Wu, Y.S. Inhibition of Rac-1 activity by NSC23766 prevents cartilage endplate degeneration via Wnt/β-catenin pathway. J. Cell. Mol. Med. 2020, 24, 3582–3592. [Google Scholar] [CrossRef]
- Hu, L.; Luo, D.; Zhang, H.; He, L. Polydatin inhibits IL-1β-mediated chondrocyte inflammation and ameliorates cartilage degradation: Involvement of the NF-κB and Wnt/β-catenin pathways. Tissue Cell 2022, 78, 101865. [Google Scholar] [CrossRef]
- Takase, K.; McCulloch, P.C.; Yik, J.H.N.; Haudenschild, D.R. Clinical and molecular landscape of post-traumatic osteoarthritis. Connect. Tissue Res. 2025, 66, 373–379. [Google Scholar] [CrossRef]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3, 96308. [Google Scholar] [CrossRef]
- Reis, C.V.; Andrade, S.A.; Ramos, O.H.; Ramos, C.R.; Ho, P.L.; Batista, I.F.; Chudzinski-Tavassi, A.M. Lopap, a prothrombin activator from Lonomia obliqua belonging to the lipocalin family: Recombinant production, biochemical characterization and structure-function insights. Biochem. J. 2006, 398, 295–302. [Google Scholar] [CrossRef]
- Alvarez-Flores, M.P.; Furlin, D.; Ramos, O.H.; Balan, A.; Konno, K.; Chudzinski-Tavassi, A.M. Losac, the first hemolin that exhibits procogulant activity through selective factor X proteolytic activation. J. Biol. Chem. 2011, 286, 6918–6928. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Neun, B.W.; Clogston, J.D.; Grossman, J.H.; McNeil, S.E. Choice of method for endotoxin detection depends on nanoformulation. Nanomedicine 2014, 9, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.; Prina-Mello, A. Endotoxin contamination of engineered nanomaterials: Overcoming the hurdles associated with endotoxin testing. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1738. [Google Scholar] [CrossRef] [PubMed]
- Chaiwut, R.; Kasinrerk, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Res. Notes 2022, 15, 42. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef]
- Bevc, K.; Zhang, S.; Pazos-Perez, A.; Alonso-Perez, A.; Fercher, D.; Kauppinen, S.; Frondelius, T.; Bruhin, V.; Salzmann, G.; Rauer, T.; et al. Evaluating the role of lipopolysaccharides in the joint: Fibronectin as a novel protective mechanism. RMD Open 2025, 11, e005622. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef]
- Shen, J.; Xu, S.; Zhou, H.; Liu, H.; Jiang, W.; Hao, J.; Hu, Z. IL-1β induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci. Rep. 2017, 7, 41067. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Woods, A.; Wang, G.; Dupuis, H.; Shao, Z.; Beier, F. Rac-1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J. Biol. Chem. 2007, 282, 23500–23508. [Google Scholar] [CrossRef]
- Marei, H.; Malliri, A. Rac-1 in human diseases: The therapeutic potential of targeting Rac-1 signaling regulatory mechanisms. Small GTPases 2017, 8, 139–163. [Google Scholar] [CrossRef]
- Han, G.; Li, C.; Zhang, N.; Liu, Q.; Huang, L.; Xia, Y.; Xu, J. CmHem, a hemolin-like gene identified from Cnaphalocrocis medinalis, involved in metamorphosis and baculovirus infection. PeerJ 2023, 11, e16225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, H.; Wu, Y.; Heng, B.C.; Chen, P.; Liu, H.; Ouyang, H.W. Wnt and Rho GTPase signaling in osteoarthritis development and intervention: Implications for diagnosis and therapy. Arthritis Res. Ther. 2013, 15, 217. [Google Scholar] [CrossRef]
- Öztürk, E.; Despot-Slade, E.; Pichler, M.; Zenobi-Wong, M. RhoA activation and nuclearization marks loss of chondrocyte phenotype in crosstalk with Wnt pathway. Exp. Cell Res. 2017, 360, 113–124. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Ballesteros-Mejia, L.; Díaz-Díaz, J.; Toro-Vargas, D.M.; Amarillo-Suarez, A.R.; Gey, D.; León, C.; Tovar, E.; Arias, M.; Rivera, N.; et al. Deadly and venomous Lonomia caterpillars are more than the two usual suspects. PLoS Neglected Trop. Dis. 2023, 17, e0011063. [Google Scholar] [CrossRef]
- Illescas, M.; Peñas, A.; Arenas, J.; Martín, M.A.; Ugalde, C. Regulation of Mitochondrial Function by the Actin Cytoskeleton. Front. Cell Dev. Biol. 2021, 9, 795838. [Google Scholar] [CrossRef] [PubMed]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]
- Heinen, T.E.; de Farias, C.B.; Abujamra, A.L.; Mendonça, R.Z.; Roesler, R.; da Veiga, A.B. Effects of Lonomia obliqua caterpillar venom upon the proliferation and viability of cell lines. Cytotechnology 2014, 66, 63–74. [Google Scholar] [CrossRef]
- de Almeida Schneider, R.; Barros Terraciano, P.; Zanon, P.; Quandt, L.; Zanini Gotardi, D.H.; Alves Garcez, T.N.; Santi, L.; Beys da Silva, W.O.; Sereno Montenegro, I.; Yates, J.; et al. Mechanisms involved in the cytoprotective effects of Lonomia obliqua venom on human endometrial stromal cells. Toxicon 2024, 240, 107630. [Google Scholar] [CrossRef]
- Oliveira, D.S.; de Souza, J.G.; Alvarez-Flores, M.P.; Cunegundes, P.S.; DeOcesano-Pereira, C.; Lobba, A.M.; Gomes, R.N.; Chudzinski-Tavassi, A.M. Lonomia obliqua Venom Induces NF-κB Activation and a Pro-Inflammatory Profile in THP-1-Derived Macrophage. Toxins 2021, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.F.; Dragulev, B.; Guimarães, J.A.; Fox, J.W. Novel perspectives on the pathogenesis of Lonomia obliqua caterpillar envenomation based on assessment of host response by gene expression analysis. Toxicon 2008, 51, 1119–1128. [Google Scholar] [CrossRef]
- Fritzen, M.; Flores, M.P.; Reis, C.V.; Chudzinski-Tavassi, A.M. A prothrombin activator (Lopap) modulating inflammation, coagulation and cell survival mechanisms. Biochem. Biophys. Res. Commun. 2005, 333, 517–523. [Google Scholar] [CrossRef]
- Waismam, K.; Chudzinski-Tavassi, A.M.; Carrijo-Carvalho, L.C.; Pacheco, M.T.; Farsky, S.H. Lopap: A non-inflammatory and cytoprotective molecule in neutrophils and endothelial cells. Toxicon 2009, 53, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahrari, S.; Salimian, J.; Salehi, Z.; Karimi, M.; Emamvirdizadeh, A.; Jamalkandi, S.A.; Ghanei, M. p38 MAPK signaling in chronic obstructive pulmonary disease pathogenesis and inhibitor therapeutics. Cell Commun. Signal. 2023, 21, 314. [Google Scholar] [CrossRef]
- Jin, S.; Mutvei, A.P.; Chivukula, I.V.; Andersson, E.R.; Ramsköld, D.; Sandberg, R.; Lee, K.L.; Kronqvist, P.; Mamaeva, V.; Ostling, P.; et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene 2013, 32, 4892–4902. [Google Scholar] [CrossRef]
- Brakebusch, C. Rho GTPase Signaling in Health and Disease: A Complex Signaling Network. Cells 2021, 10, 401. [Google Scholar] [CrossRef]
- Wang, G.; Beier, F. Rac-1/Cdc42 and RhoA GTPases antagonistically regulate chondrocyte proliferation, hypertrophy, and apoptosis. J. Bone Miner. Res. 2005, 20, 1022–1031. [Google Scholar] [CrossRef]
- Wang, G.; Woods, A.; Agoston, H.; Ulici, V.; Glogauer, M.; Beier, F. Genetic ablation of Rac-1 in cartilage results in chondrodysplasia. Dev. Biol. 2007, 306, 612–623. [Google Scholar] [CrossRef]
- Yu, O.M.; Brown, J.H. G Protein-Coupled Receptor and RhoA-Stimulated Transcriptional Responses: Links to Inflammation, Differentiation, and Cell Proliferation. Mol. Pharmacol. 2015, 88, 171–180. [Google Scholar] [CrossRef]
- Kucera, A.; Bakke, O.; Progida, C. The multiple roles of Rab9 in the endolysosomal system. Commun. Integr. Biol. 2016, 9, e1204498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tang, M.; Wu, Z.; Lin, Y.; Wu, C.; Huang, H.; Chen, J.; Zhu, Z.; Liu, Y.; Tang, S.; et al. Increased Rab1a accelerates osteoarthritis by inhibiting autophagy via activation of the mTORC1-S6K pathway. J. Adv. Res. 2025, 75, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, T.; Otani, T.; Koike, T.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: Its possible role in joint degeneration. Lab. Investig. 2008, 88, 264–274. [Google Scholar] [CrossRef]
- van Donkelaar, C.C.; Wilson, W. Mechanics of chondrocyte hypertrophy. Biomech. Model. Mechanobiol. 2012, 11, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Mainardi, A.; Majumder, N.; Dönges, L.; Kumar, B.; Occhetta, P.; Martin, I.; Egloff, C.; Ghosh, S.; Bandyopadhyay, A.; et al. Chondrocyte Hypertrophy in Osteoarthritis: Mechanistic Studies and Models for the Identification of New Therapeutic Strategies. Cells 2022, 11, 4034. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Alvarez-Flores, M.P.; de Melo, A.T.; Gomes, R.N.; de Melo, T.C.; Oliveira, D.S.; de Souza, M.M.; DeOcesano-Pereira, C.; Goldfeder, M.B.; Faria, F.; Chudzinski-Tavassi, A.M. Venom-Derived Proteins from Lonomia obliqua Modulate Cytoskeletal Regulators and Inflammatory Responses in Human Chondrocytes. Int. J. Mol. Sci. 2026, 27, 934. https://doi.org/10.3390/ijms27020934
Alvarez-Flores MP, de Melo AT, Gomes RN, de Melo TC, Oliveira DS, de Souza MM, DeOcesano-Pereira C, Goldfeder MB, Faria F, Chudzinski-Tavassi AM. Venom-Derived Proteins from Lonomia obliqua Modulate Cytoskeletal Regulators and Inflammatory Responses in Human Chondrocytes. International Journal of Molecular Sciences. 2026; 27(2):934. https://doi.org/10.3390/ijms27020934
Chicago/Turabian StyleAlvarez-Flores, Miryam Paola, Amanda Teixeira de Melo, Renata Nascimento Gomes, Thatiana Corrêa de Melo, Douglas Souza Oliveira, Marcelo Medina de Souza, Carlos DeOcesano-Pereira, Mauricio Barbugiani Goldfeder, Fernanda Faria, and Ana Marisa Chudzinski-Tavassi. 2026. "Venom-Derived Proteins from Lonomia obliqua Modulate Cytoskeletal Regulators and Inflammatory Responses in Human Chondrocytes" International Journal of Molecular Sciences 27, no. 2: 934. https://doi.org/10.3390/ijms27020934
APA StyleAlvarez-Flores, M. P., de Melo, A. T., Gomes, R. N., de Melo, T. C., Oliveira, D. S., de Souza, M. M., DeOcesano-Pereira, C., Goldfeder, M. B., Faria, F., & Chudzinski-Tavassi, A. M. (2026). Venom-Derived Proteins from Lonomia obliqua Modulate Cytoskeletal Regulators and Inflammatory Responses in Human Chondrocytes. International Journal of Molecular Sciences, 27(2), 934. https://doi.org/10.3390/ijms27020934






