Blood–Nerve Barrier Breakdown Induced by Immunoglobulin G in Typical and Multifocal Chronic Inflammatory Demyelinating Polyneuropathy and Multifocal Motor Neuropathy
Abstract
1. Introduction
2. Results
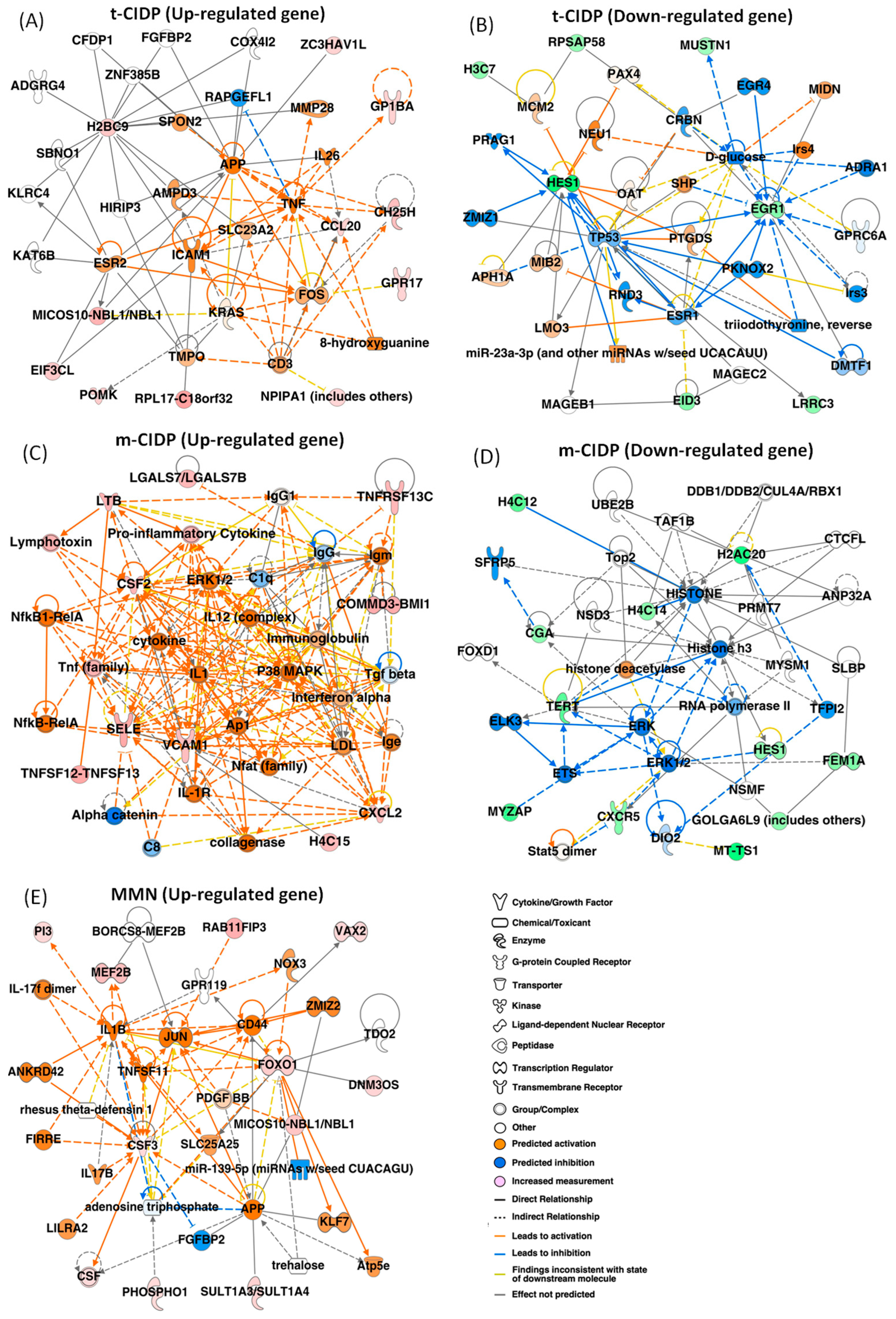
2.1. Identification of the Changed Gene Expression in FH-BNB Cells After Exposure to IgG from Typical CIDP, Multifocal CIDP, or MMN Patients by RNA-seq
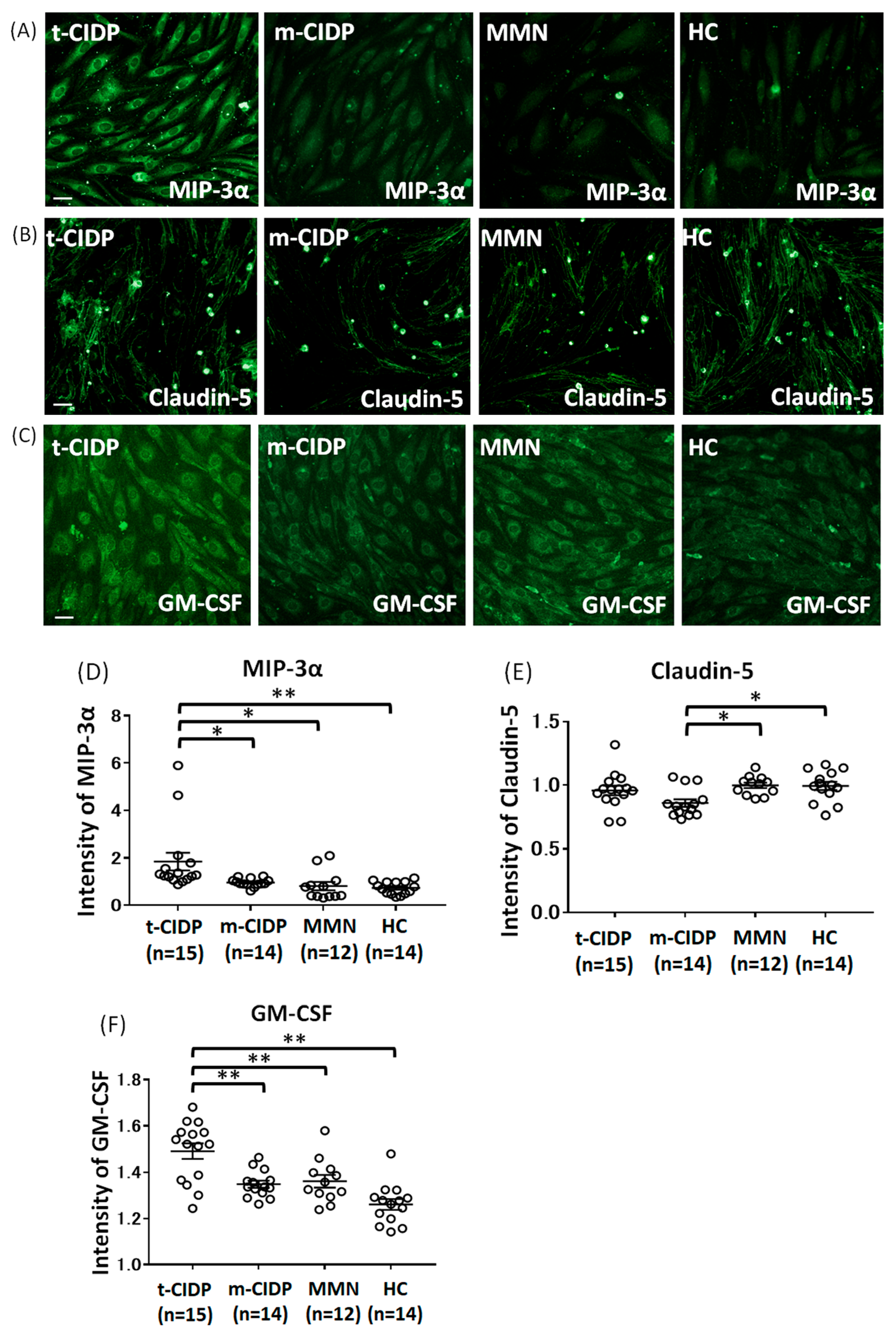
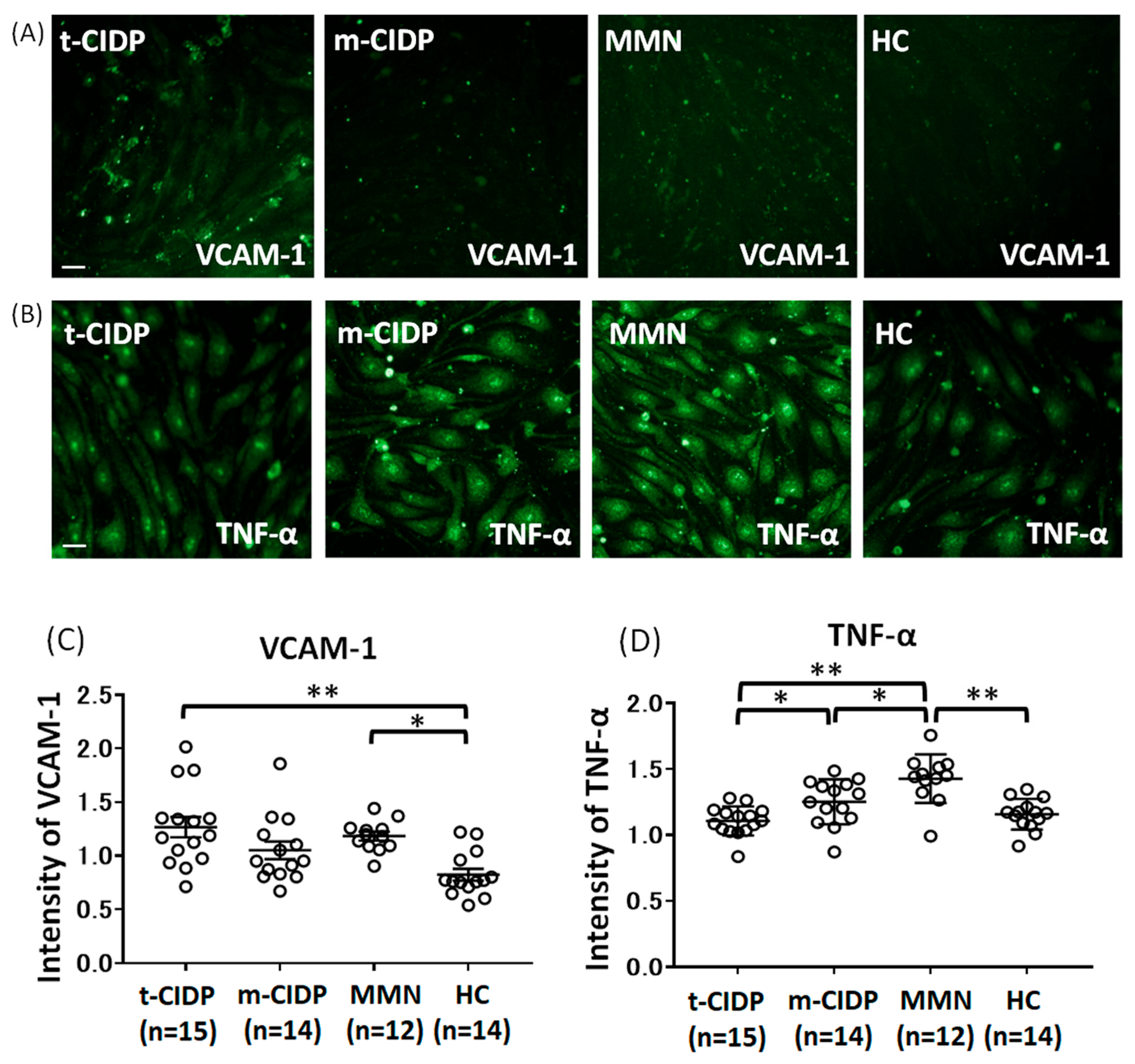
2.2. Change in MIP-3α, Claudin-5, GM-CSF, VCAM-1, TNF-α, ICAM-1, and IP-10 in BNB-Endothelial Cells After Exposure to Patient IgG
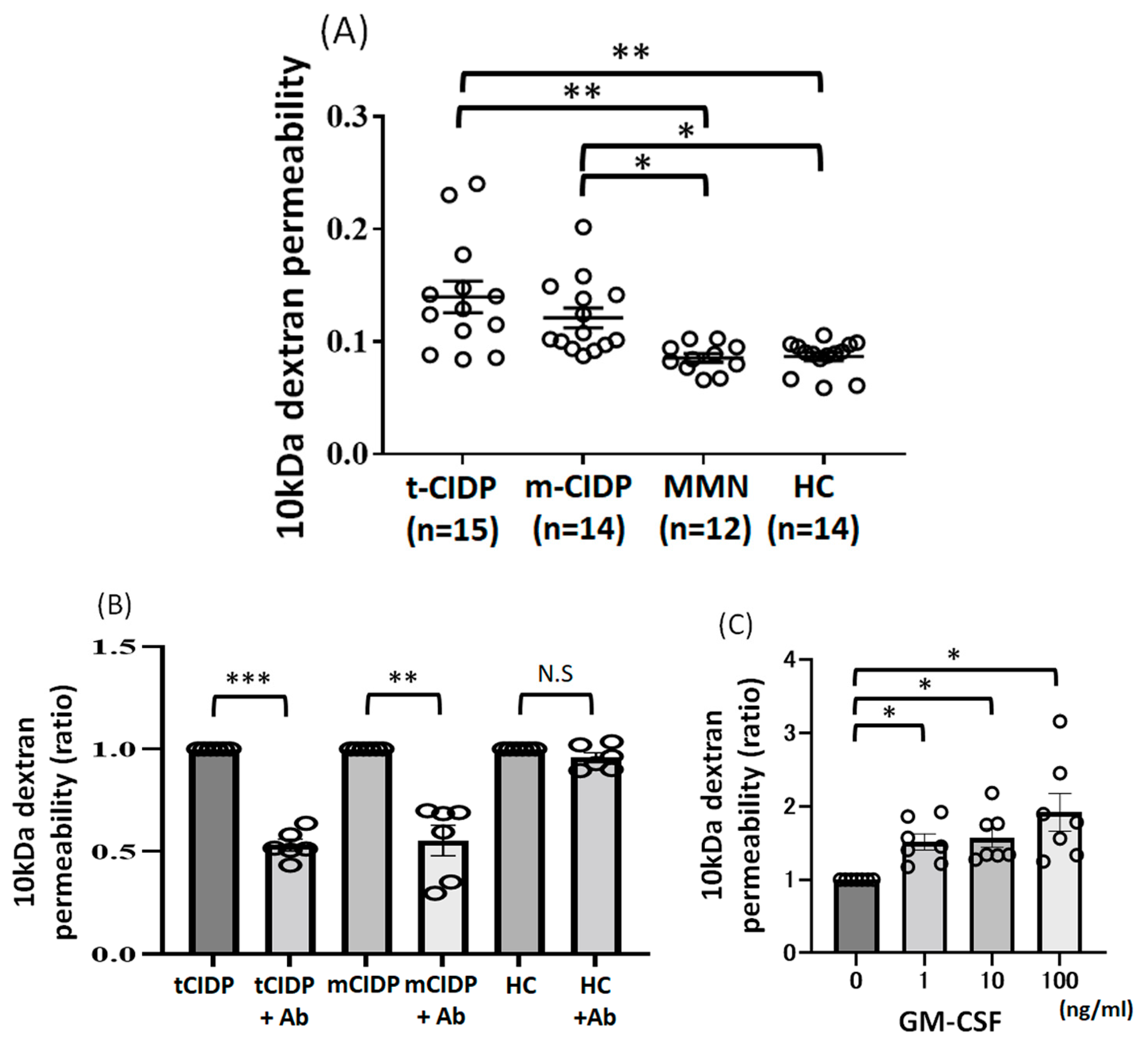
2.3. Change of 10 kDa-Dextran Permeability in BNB-Endothelial Cells After Exposure to IgG from Typical CIDP, Multifocal CIDP, and MMN Patients and HCs
3. Discussion
4. Methods
4.1. Study Population
4.2. Whole Transcriptome Analyses with RNA-seq
4.3. Immunohistochemistry of MIP-3α, Claudin-5, GM-CSF, VCAM-1, TNF-α, ICAM-1, and IP-10 Through the High-Content Imaging Assay
4.4. Paracellular Permeability of 10 kDa Dextran
4.5. Treatment with GM-CSF Neutralizing Antibodies or GM-CSF
4.6. Treatment with GM-CSF
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehmann, H.C.; Burke, D.; Kuwabara, S. Chronic inflammatory demyelinating polyneuropathy: Update on diagnosis, immunopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2019, 90, 981–987. [Google Scholar] [CrossRef]
- Mathey, E.K.; Park, S.B.; Hughes, R.A.; Pollard, J.D.; Armati, P.J.; Barnett, M.H.; Taylor, B.V.; Dyck, P.J.; Kiernan, M.C.; Lin, C.S. Chronic inflammatory demyelinating polyradiculoneuropathy: From pathology to phenotype. J. Neurol. Neurosurg. Psychiatry 2015, 86, 973–985. [Google Scholar] [CrossRef]
- Dalakas, M.C. Pathogenesis of immune-mediated neuropathies. Biochim. Biophys. Acta 2015, 1852, 658–666. [Google Scholar] [CrossRef]
- Rajabally, Y.A. Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Current Therapeutic Approaches and Future Outlooks. Immunotargets Ther. 2024, 13, 99–110. [Google Scholar] [CrossRef]
- Van den Bergh, P.Y.K.; van Doorn, P.A.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Allen, J.A.; Attarian, S.; Blomkwist-Markens, P.H.; Cornblath, D.R.; Eftimov, F.; et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. J. Peripher. Nerv. Syst. 2021, 26, 242–268. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, S.; Isose, S.; Mori, M.; Mitsuma, S.; Sawai, S.; Beppu, M.; Sekiguchi, Y.; Misawa, S. Different electrophysiological profiles and treatment response in ‘typical’ and ‘atypical’ chronic inflammatory demyelinating polyneuropathy. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1054–1059. [Google Scholar] [CrossRef]
- Vlam, L.; van der Pol, W.L.; Cats, E.A.; Straver, D.C.; Piepers, S.; Franssen, H.; van den Berg, L.H. Multifocal motor neuropathy: Diagnosis, pathogenesis and treatment strategies. Nat. Rev. Neurol. 2011, 8, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.Z.; Dyck, P.J.; van den Berg, L.H.; Kiernan, M.C.; Taylor, B.V. Multifocal motor neuropathy: Controversies and priorities. J. Neurol. Neurosurg. Psychiatry 2020, 91, 140–148. [Google Scholar] [CrossRef]
- Shimizu, F.; Sawai, S.; Sano, Y.; Beppu, M.; Misawa, S.; Nishihara, H.; Koga, M.; Kuwabara, S.; Kanda, T. Severity and patterns of blood-nerve barrier breakdown in patients with chronic inflammatory demyelinating polyradiculoneuropathy: Correlations with clinical subtypes. PLoS ONE 2014, 9, E104205. [Google Scholar] [CrossRef]
- Shimizu, F.; Oishi, M.; Sawai, S.; Beppu, M.; Misawa, S.; Matsui, N.; Miyashiro, A.; Maeda, T.; Takeshita, Y.; Nishihara, H.; et al. Increased IP-10 production by blood-nerve barrier in multifocal acquired demyelinating sensory and motor neuropathy and multifocal motor neuropathy. J. Neurol. Neurosurg. Psychiatry 2019, 90, 444–450. [Google Scholar] [CrossRef]
- Shimizu, F.; Omoto, M.; Sano, Y.; Mastui, N.; Miyashiro, A.; Tasaki, A.; Maeda, T.; Koga, M.; Kaji, R.; Kanda, T. Sera from patients with multifocal motor neuropathy disrupt the blood-nerve barrier. J. Neurol. Neurosurg. Psychiatry 2014, 85, 526–537. [Google Scholar] [CrossRef]
- Kanda, T. Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J. Neurol. Neurosurg. Psychiatry 2013, 84, 208–212. [Google Scholar] [CrossRef]
- Kanda, T.; Numata, Y.; Mizusawa, H. Chronic inflammatory demyelinating polyneuropathy: Decreased claudin-5 and relocated ZO-1. J. Neurol. Neurosurg. Psychiatry 2004, 75, 765–769. [Google Scholar] [CrossRef]
- Kaji, R.; Oka, N.; Tsuji, T.; Mezaki, T.; Nishio, T.; Akiguchi, I.; Kimura, J. Pathological findings at the site of conduction block in multifocal motor neuropathy. Ann. Neurol. 1993, 33, 152–158. [Google Scholar] [CrossRef]
- Bunschoten, C.; Jacobs, B.C.; Van den Bergh, P.Y.K.; Cornblath, D.R.; van Doorn, P.A. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019, 18, 784–794. [Google Scholar] [CrossRef]
- Heininger, K.; Liebert, U.G.; Toyka, K.V.; Haneveld, F.T.; Schwendemann, G.; Kolb-Bachofen, V.; Ross, H.G.; Cleveland, S.; Besinger, U.A.; Gibbels, E.; et al. Chronic inflammatory polyneuropathy. Reduction of nerve conduction velocities in monkeys by systemic passive transfer of immunoglobulin G. J. Neurol. Sci. 1984, 66, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.X.; Taylor, J.; Andrias-Kauba, S.; Pollard, J.D. Passive transfer of demyelination by serum or IgG from chronic inflammatory demyelinating polyneuropathy patients. Ann. Neurol. 2000, 47, 765–775. [Google Scholar] [CrossRef]
- Allen, J.A.; Lin, J.; Basta, I.; Dysgaard, T.; Eggers, C.; Guptill, J.T.; Gwathmey, K.G.; Hewamadduma, C.; Hofman, E.; Hussain, Y.M.; et al. Safety, tolerability, and efficacy of subcutaneous efgartigimod in patients with chronic inflammatory demyelinating polyradiculoneuropathy (ADHERE): A multicentre, randomised-withdrawal, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2024, 23, 1013–1024, Erratum in Lancet Neurol. 2025, 24, e8. [Google Scholar] [CrossRef] [PubMed]
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun. Rev. 2021, 20, 102846. [Google Scholar] [CrossRef]
- Lee, A.Y.; Eri, R.; Lyons, A.B.; Grimm, M.C.; Korner, H. CC Chemokine Ligand 20 and Its Cognate Receptor CCR6 in Mucosal T Cell Immunology and Inflammatory Bowel Disease: Odd Couple or Axis of Evil? Front. Immunol. 2013, 4, 194. [Google Scholar] [CrossRef] [PubMed]
- Restorick, S.M.; Durant, L.; Kalra, S.; Hassan-Smith, G.; Rathbone, E.; Douglas, M.R.; Curnow, S.J. CCR6+ Th cells in the cerebrospinal fluid of persons with multiple sclerosis are dominated by pathogenic non-classic Th1 cells and GM-CSF-only-secreting Th cells. Brain Behav. Immun. 2017, 64, 71–79. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Yuan, Z.R.; Tan, H.S.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Paré, A.; Mailhot, B.; Lévesque, S.A.; Juzwik, C.; Ignatius Arokia Doss, P.M.; Lécuyer, M.A.; Prat, A.; Rangachari, M.; Fournier, A.; Lacroix, S. IL-1β enables CNS access to CCR2hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1194–E1203. [Google Scholar] [CrossRef]
- Shang, S.; Yang, Y.M.; Zhang, H.; Tian, L.; Jiang, J.S.; Dong, Y.B.; Zhang, K.; Li, B.; Zhao, W.D.; Fang, W.G.; et al. Intracerebral GM-CSF contributes to transendothelial monocyte migration in APP/PS1 Alzheimer’s disease mice. J. Cereb. Blood Flow Metab. 2016, 36, 1978–1991. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Matsui, N.; Fujita, K.; Miyashiro, A.; Nodera, H.; Izumi, Y.; Shimizu, F.; Miyamoto, K.; Takahashi, Y.; Kanda, T.; et al. Increased proinflammatory cytokines in sera of patients with multifocal motor neuropathy. J. Neurol. Sci. 2014, 346, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First revision. J. Peripher. Nerv. Syst. 2010, 15, 295–301. [Google Scholar] [CrossRef]
- Ludolph, A.; Drory, V.; Hardiman, O.; Nakano, I.; Ravits, J.; Robberecht, W.; Shefner, J. WFN Research Group On ALS/MND. A revision of the El Escorial criteria—2015. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 291–292. [Google Scholar] [CrossRef]
- Abe, M.; Sano, Y.; Maeda, T.; Shimizu, F.; Kashiwamura, Y.; Haruki, H.; Saito, K.; Tasaki, A.; Kawai, M.; Terasaki, T.; et al. Establishment and characterization of human peripheral nerve microvascular endothelial cell lines: A new in vitro blood-nerve barrier (BNB) model. Cell Struct. Funct. 2012, 37, 89–100. [Google Scholar] [CrossRef]
- Kohno, M.; Kobayashi, S.; Yamamoto, T.; Yoshitomi, R.; Kajii, T.; Fujii, S.; Nakamura, Y.; Kato, T.; Uchinoumi, H.; Oda, T.; et al. Enhancing calmodulin binding to cardiac ryanodine receptor completely inhibits pressure-overload induced hypertrophic signaling. Commun. Biol. 2020, 3, 714. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Takeshita, Y.; Sano, Y.; Hamamoto, Y.; Shiraishi, H.; Sato, T.; Yoshimura, S.; Maeda, T.; Fujikawa, S.; Nishihara, H.; et al. GRP78 antibodies damage the blood-brain barrier and relate to cerebellar degeneration in Lambert-Eaton myasthenic syndrome. Brain 2019, 142, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Koga, M.; Mizukami, Y.; Watanabe, K.; Sato, R.; Takeshita, Y.; Maeda, T.; Kanda, T.; Nakamori, M. Small Nuclear Ribonucleoprotein Autoantibody Associated With Blood-Nerve Barrier Breakdown in Guillain-Barré Syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200405. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shimizu, F.; Sato, R.; Mizukami, Y.; Watanabe, K.; Maeda, T.; Kanda, T.; Matsui, N.; Misawa, S.; Izumi, Y.; Kuwabara, S.; et al. Blood–Nerve Barrier Breakdown Induced by Immunoglobulin G in Typical and Multifocal Chronic Inflammatory Demyelinating Polyneuropathy and Multifocal Motor Neuropathy. Int. J. Mol. Sci. 2026, 27, 1088. https://doi.org/10.3390/ijms27021088
Shimizu F, Sato R, Mizukami Y, Watanabe K, Maeda T, Kanda T, Matsui N, Misawa S, Izumi Y, Kuwabara S, et al. Blood–Nerve Barrier Breakdown Induced by Immunoglobulin G in Typical and Multifocal Chronic Inflammatory Demyelinating Polyneuropathy and Multifocal Motor Neuropathy. International Journal of Molecular Sciences. 2026; 27(2):1088. https://doi.org/10.3390/ijms27021088
Chicago/Turabian StyleShimizu, Fumitaka, Ryota Sato, Yoichi Mizukami, Kenji Watanabe, Toshihiko Maeda, Takashi Kanda, Naoko Matsui, Sonoko Misawa, Yuishin Izumi, Satoshi Kuwabara, and et al. 2026. "Blood–Nerve Barrier Breakdown Induced by Immunoglobulin G in Typical and Multifocal Chronic Inflammatory Demyelinating Polyneuropathy and Multifocal Motor Neuropathy" International Journal of Molecular Sciences 27, no. 2: 1088. https://doi.org/10.3390/ijms27021088
APA StyleShimizu, F., Sato, R., Mizukami, Y., Watanabe, K., Maeda, T., Kanda, T., Matsui, N., Misawa, S., Izumi, Y., Kuwabara, S., & Nakamori, M. (2026). Blood–Nerve Barrier Breakdown Induced by Immunoglobulin G in Typical and Multifocal Chronic Inflammatory Demyelinating Polyneuropathy and Multifocal Motor Neuropathy. International Journal of Molecular Sciences, 27(2), 1088. https://doi.org/10.3390/ijms27021088





