Cortical Neuroprotective Mechanisms of Exercise Training in Post-Traumatic Brain Injury: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Risk of Bias
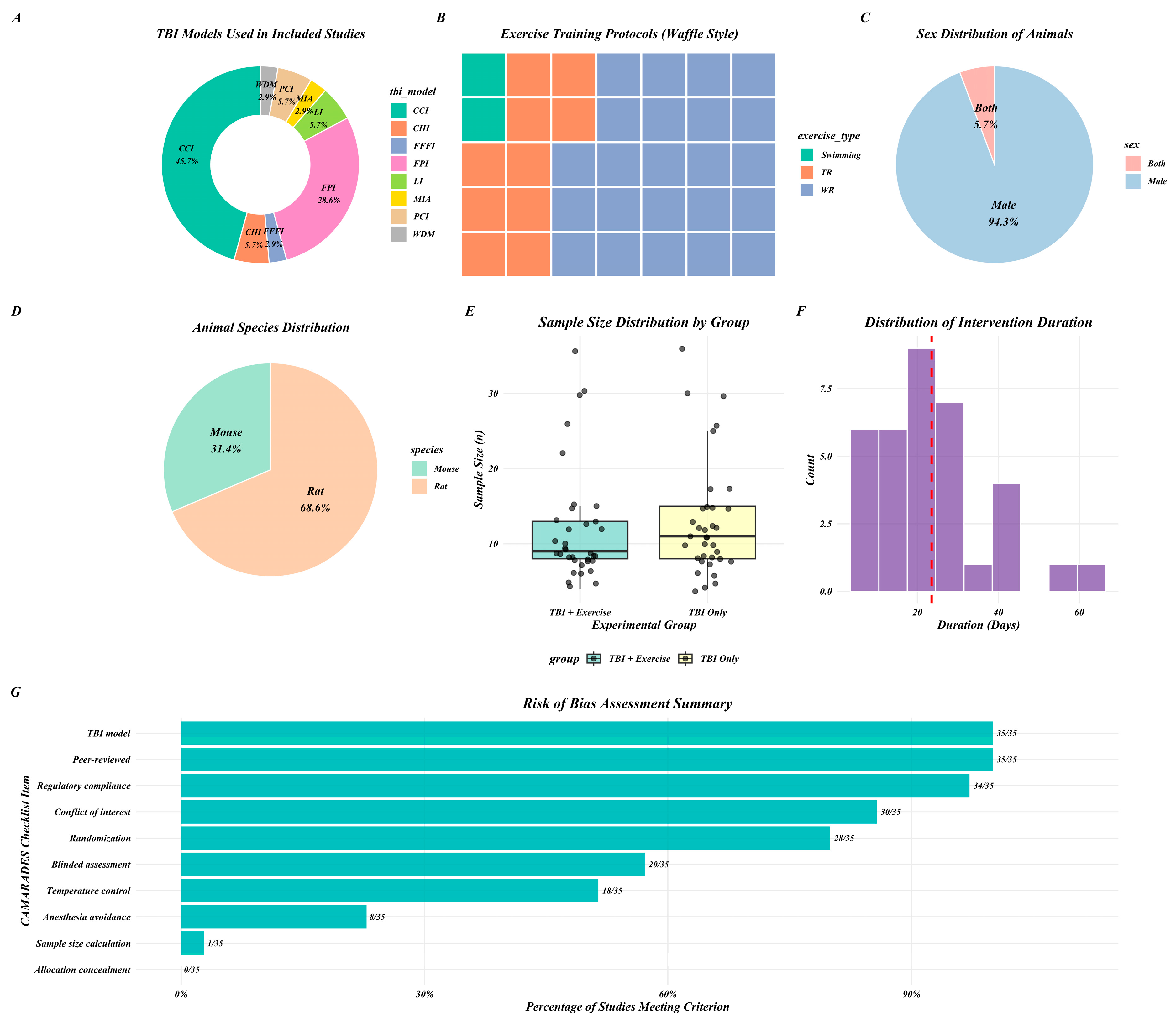
3. Results
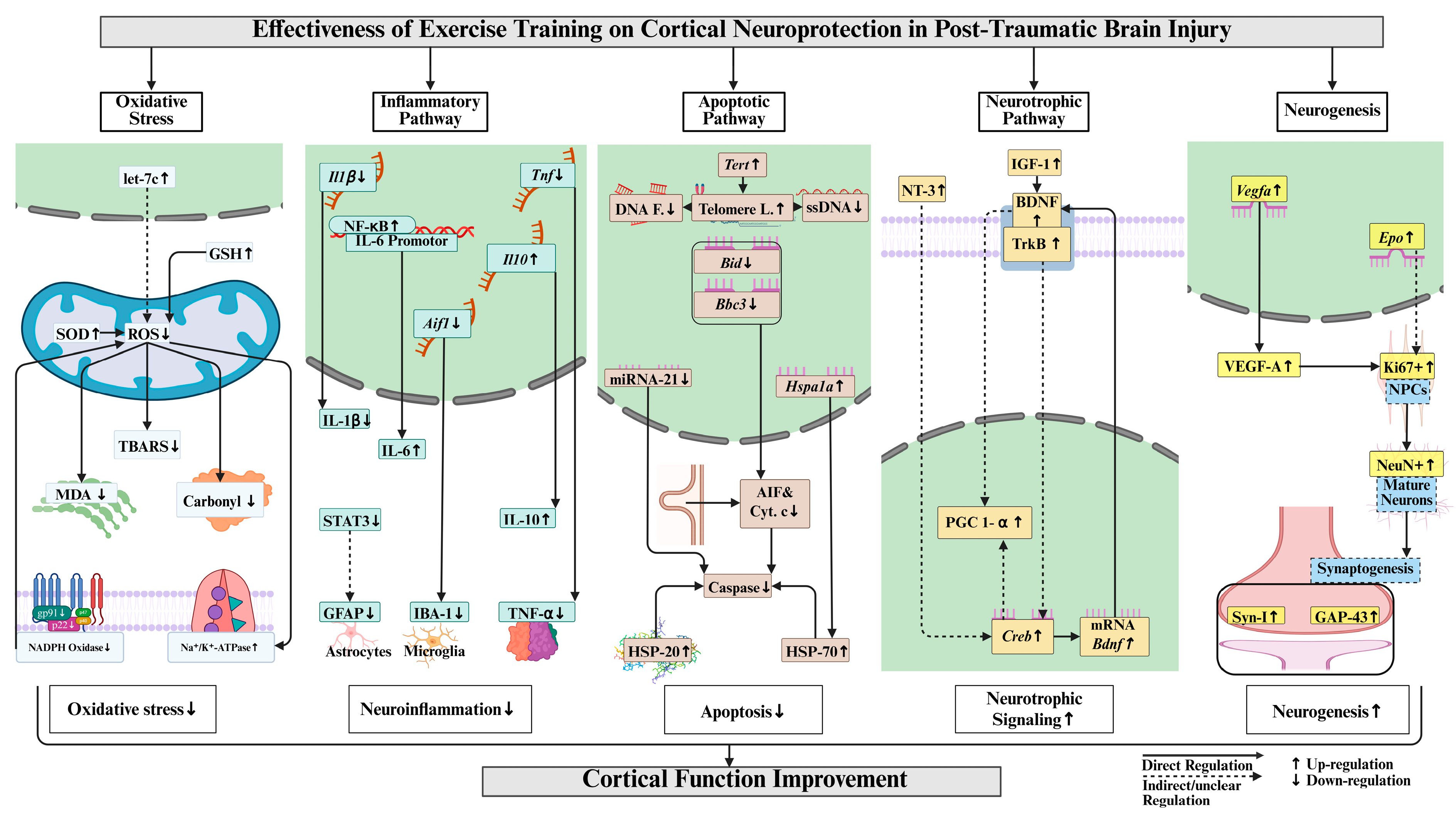
3.1. Oxidative Stress
3.2. Inflammatory Pathway
3.3. Apoptotic Pathway
3.4. Mitochondrial Function
3.5. Neurotrophic Factors
3.6. Neurogenesis
3.7. Motor Function
4. Discussion
Limitations and Future Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
PROSPERO Registration No
References
- Corrigan, F.; Wee, I.C.; Collins-Praino, L.E. Chronic motor performance following different traumatic brain injury severity-A systematic review. Front. Neurol. 2023, 14, 1180353. [Google Scholar] [CrossRef]
- Nelson, L.D.; Temkin, N.R.; Dikmen, S.; Barber, J.; Giacino, J.T.; Yuh, E.; Levin, H.S.; McCrea, M.A.; Stein, M.B.; Mukherjee, P.; et al. Recovery After Mild Traumatic Brain Injury in Patients Presenting to US Level I Trauma Centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA Neurol. 2019, 76, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Singh, T.G.; Kaur, A. Traumatic Brain Injury: Mechanistic Insight on Pathophysiology and Potential Therapeutic Targets. J. Mol. Neurosci. 2021, 71, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Suman, P.; Paul, A.; Mishra, A. Pathophysiology and Management Approaches for Traumatic Brain Injury. In Drug Delivery Strategies in Neurological Disorders: Challenges and Opportunities; Mishra, A., Kulhari, H., Eds.; Springer Nature: Singapore, 2023; pp. 173–188. [Google Scholar]
- Itoh, T.; Imano, M.; Nishida, S.; Tsubaki, M.; Hashimoto, S.; Ito, A.; Satou, T. Exercise inhibits neuronal apoptosis and improves cerebral function following rat traumatic brain injury. J. Neural Transm. 2011, 118, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Mota, B.C.; Pereira, L.; Souza, M.A.; Silva, L.F.; Magni, D.V.; Ferreira, A.P.; Oliveira, M.S.; Furian, A.F.; Mazzardo-Martins, L.; Silva, M.D.; et al. Exercise pre-conditioning reduces brain inflammation and protects against toxicity induced by traumatic brain injury: Behavioral and neurochemical approach. Neurotox. Res. 2012, 21, 175–184. [Google Scholar] [CrossRef]
- Rafie, F.; Khaksari, M.; Amiresmaili, S.; Soltani, Z.; Pourranjbar, M.; Shirazpour, S.; Jafari, E. Protective effects of early exercise on neuroinflammation, and neurotoxicity associated by traumatic brain injury: A behavioral and neurochemical approach. Int. J. Neurosci. 2024, 134, 700–713. [Google Scholar] [CrossRef]
- Jha, S.; Ghewade, P. Management and Treatment of Traumatic Brain Injuries. Cureus 2022, 14, e30617. [Google Scholar] [CrossRef]
- Mendes Arent, A.; Souza, L.F.d.; Walz, R.; Dafre, A.L. Perspectives on Molecular Biomarkers of Oxidative Stress and Antioxidant Strategies in Traumatic Brain Injury. BioMed Res. Int. 2014, 2014, 723060. [Google Scholar] [CrossRef]
- Amlerova, Z.; Chmelova, M.; Anderova, M.; Vargova, L. Reactive gliosis in traumatic brain injury: A comprehensive review. Front. Cell. Neurosci. 2024, 18, 1335849. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Wang, R.; Brann, D.W. NADPH oxidases in traumatic brain injury—Promising therapeutic targets? Redox Biol. 2018, 16, 285–293. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191, Erratum in Nat. Rev. Neurol. 2017, 13, 572.. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Z.; Zhang, L.; Yang, X.; Zhu, J.; Zhou, X.; Liao, X. Esketamine attenuates traumatic brain injury by modulating STAT3-mediated Glycolysis and immune responses. BMC Neurosci. 2025, 26, 21. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.C.; Lin, H.J.; Tian, Y.F.; Chen, Y.C.; Lin, M.T.; Lin, C.H.; Chang, C.P.; Hsu, C.C. Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-ΚB/IL-6/synapsin I axis in traumatic brain injury rats. J. Neuroinflamm. 2017, 14, 90. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Hanafy, K.A. Cell Death and Recovery in Traumatic Brain Injury. Neurotherapeutics 2020, 17, 446–456. [Google Scholar] [CrossRef]
- Hakiminia, B.; Alikiaii, B.; Khorvash, F.; Mousavi, S. Oxidative stress and mitochondrial dysfunction following traumatic brain injury: From mechanistic view to targeted therapeutic opportunities. Fundam. Clin. Pharmacol. 2022, 36, 612–662. [Google Scholar] [CrossRef]
- Zhou, J.; Shen, R.; Makale, E.C.; Zhong, W.; Chen, Z.; Huang, Q. SS31 Confers Cerebral Protection by Reversing Mitochondrial Dysfunction in Early Brain Injury Following Subarachnoid Hemorrhage, via the Nrf2- and PGC-1α-Dependent Pathways. Neurochem. Res. 2023, 48, 1580–1595. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Wang, L.; Li, Z.; Tang, S.; Wang, Y.; Gu, N.; Sun, X.; Li, L. TREM2 activation alleviates neural damage via Akt/CREB/BDNF signalling after traumatic brain injury in mice. J. Neuroinflamm. 2022, 19, 289. [Google Scholar] [CrossRef]
- Dai, X.-Y.; Song, W.-L.; Wu, J.-Y.; Gao, S.-J.; Liu, L.; Wu, H.; Zhang, L.-Q.; Liu, D.-Q.; Zhou, Y.-Q.; Tang, Y.-X.; et al. The role of PGC-1α in brain injury: Mechanisms and therapeutic potential. Brain Hemorrhages 2025. [Google Scholar] [CrossRef]
- Lange, C.; Storkebaum, E.; de Almodóvar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef]
- Celorrio, M.; Rhodes, J.; Shumilov, K.; Moritz, J.; Xiao, S.; Anabayan, I.; Sauerbeck, A.; Kummer, T.; Friess, S. Recombinant human erythropoietin induces neuroprotection, activates MAPK/CREB pathway, and rescues fear memory after traumatic brain injury with delayed hypoxemia in mice. Brain Res. 2022, 1795, 148074. [Google Scholar] [CrossRef] [PubMed]
- Godinho, D.B.; Fiorin, F.D.; Oliveira, M.S.; Furian, A.F.; Fighera, M.R.; Royes, L.F.F. The immunological influence of physical exercise on TBI-induced pathophysiology: Crosstalk between the spleen, gut, and brain. Neurosci. Biobehav. Rev. 2021, 130, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, F.; Kao, S.; Ishaq, S.; Lee, S.-D. Molecular regulation of exercise training on hippocampal neuroprotection in post-traumatic brain injury: A systematic review. Exp. Neurol. 2026, 397, 115587. [Google Scholar] [CrossRef]
- Papalia, W.L.; Nascimento, A.S.; Krishnan, G.; Broetto, N.; Furian, A.F.; Oliveira, M.S.; Royes, L.F.F.; Fighera, M.R. Physical Exercise as a Modulator of Vascular Pathology and Thrombin Generation to Improve Outcomes After Traumatic Brain Injury. Mol. Neurobiol. 2022, 59, 1124–1138, Erratum in Mol. Neurobiol. 2022, 59, 1139.. [Google Scholar] [CrossRef] [PubMed]
- Wogensen, E.; Malá, H.; Mogensen, J. The Effects of Exercise on Cognitive Recovery after Acquired Brain Injury in Animal Models: A Systematic Review. Neural Plast. 2015, 2015, 830871. [Google Scholar] [CrossRef]
- Tan, C.O.; Meehan, W.P.; Iverson, G.L.; Taylor, J.A. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 2014, 83, 1665–1672. [Google Scholar] [CrossRef]
- Widjaya, M.A.; Lee, S.D.; Cheng, W.C.; Wu, B.T. Effects of Exercise Training on Immune-Related Genes and Pathways in the Cortex of Animal Models of Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2024, 98, 1219–1234. [Google Scholar] [CrossRef]
- Ishaq, S.; Shah, I.A.; Lee, S.-D.; Wu, B.-T. Effects of exercise training on nigrostriatal neuroprotection in Parkinson’s disease: A systematic review. Front. Neurosci. 2025, 18, 1464168. [Google Scholar] [CrossRef]
- Ishaq, S.; Shah, I.A.; Lee, S.-D.; Wu, B.-T. Effects of exercise training on the nigrostriatal glutamatergic pathway and receptor interactions in Parkinson’s disease: A systematic review. Front. Aging Neurosci. 2025, 17, 1512278. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Q.; Hu, J. Neuroprotective Effect of Physical Activity in Ischemic Stroke: Focus on the Neurovascular Unit. Front. Cell. Neurosci. 2022, 16, 860573. [Google Scholar] [CrossRef]
- Hvid, L.G.; Langeskov-Christensen, M.; Stenager, E.; Dalgas, U. Exercise training and neuroprotection in multiple sclerosis. Lancet Neurol. 2022, 21, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Sandrow-Feinberg, H.R.; Houlé, J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, B.R.R.; Improta-Caria, A.C.; Melo, V.H.d.; De Sousa, R.A.L. Exercise-linked consequences on epilepsy. Epilepsy Behav. 2021, 121, 108079. [Google Scholar] [CrossRef]
- Tari, A.R.; Walker, T.L.; Huuha, A.M.; Sando, S.B.; Wisloff, U. Neuroprotective mechanisms of exercise and the importance of fitness for healthy brain ageing. Lancet 2025, 405, 1093–1118. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Yang, J. Contributions of the Primary Sensorimotor Cortex and Posterior Parietal Cortex to Motor Learning and Transfer. Brain Sci. 2024, 14, 1184. [Google Scholar] [CrossRef] [PubMed]
- Yip, D.W.; Awosika, A.O.; Lui, F. Physiology, Motor Cortical. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Singh, A.M.; Duncan, R.E.; Neva, J.L.; Staines, W.R. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci. Med. Rehabil. 2014, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Auboire, L.; Sennoga, C.A.; Hyvelin, J.M.; Ossant, F.; Escoffre, J.M.; Tranquart, F.; Bouakaz, A. Microbubbles combined with ultrasound therapy in ischemic stroke: A systematic review of in-vivo preclinical studies. PLoS ONE 2018, 13, e0191788. [Google Scholar] [CrossRef]
- Adkins, D.L.; Ferguson, L.; Lance, S.; Pevtsov, A.; McDonough, K.; Stamschror, J.; Jones, T.A.; Kozlowski, D.A. Combining Multiple Types of Motor Rehabilitation Enhances Skilled Forelimb Use Following Experimental Traumatic Brain Injury in Rats. Neurorehabil. Neural Repair 2015, 29, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Aguilar, L.; Portell-Cortés, I.; Costa-Miserachs, D.; Torras-Garcia, M.; Riubugent-Camps, È.; Almolda, B.; Coll-Andreu, M. The benefits of voluntary physical exercise after traumatic brain injury on rat’s object recognition memory: A comparison of different temporal schedules. Exp. Neurol. 2020, 326, 113178. [Google Scholar] [CrossRef]
- Barrett, J.P.; Aubrecht, T.G.; Smith, A.; Vaida, M.; Henry, R.J.; Doran, S.J.; Faden, A.I.; Stoica, B.A. Molecular Pathway Changes Associated with Different Post-Conditioning Exercise Interventions After Experimental TBI. J. Neurotrauma 2025, 42, 851–876. [Google Scholar] [CrossRef]
- Chen, M.F.; Huang, T.Y.; Kuo, Y.M.; Yu, L.; Chen, H.I.; Jen, C.J. Early postinjury exercise reverses memory deficits and retards the progression of closed-head injury in mice. J. Physiol. 2013, 591, 985–1000. [Google Scholar] [CrossRef]
- Chou, W.; Liu, Y.F.; Lin, C.H.; Lin, M.T.; Chen, C.C.; Liu, W.P.; Chang, C.P.; Chio, C.C. Exercise Rehabilitation Attenuates Cognitive Deficits in Rats with Traumatic Brain Injury by Stimulating the Cerebral HSP20/BDNF/TrkB Signalling Axis. Mol. Neurobiol. 2018, 55, 8602–8611. [Google Scholar] [CrossRef]
- Combs, H.L.; Jones, T.A.; Kozlowski, D.A.; Adkins, D.L. Combinatorial Motor Training Results in Functional Reorganization of Remaining Motor Cortex after Controlled Cortical Impact in Rats. J. Neurotrauma 2016, 33, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Crane, A.T.; Fink, K.D.; Smith, J.S. The effects of acute voluntary wheel running on recovery of function following medial frontal cortical contusions in rats. Restor. Neurol. Neurosci. 2012, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.R.T.; Ferreira, A.P.O.; Busanello, G.L.; da Silva, L.R.H.; da Silveira Junior, M.E.P.; Fiorin, F.D.S.; Arrifano, G.; Crespo-López, M.E.; Barcelos, R.P.; Cuevas, M.J.; et al. Previous physical exercise alters the hepatic profile of oxidative-inflammatory status and limits the secondary brain damage induced by severe traumatic brain injury in rats. J. Physiol. 2017, 595, 6023–6044. [Google Scholar] [CrossRef]
- Ferguson, L.; Giza, C.C.; Serpa, R.O.; Greco, T.; Folkerts, M.; Prins, M.L. Recovery From Repeat Mild Traumatic Brain Injury in Adolescent Rats Is Dependent on Pre-injury Activity State. Front. Neurol. 2020, 11, 616661. [Google Scholar] [CrossRef]
- Gan, T.T.; Liao, Q.; Wang, J.H.; Fan, Z.H.; Cao, J.; Pan, H.J.; Lou, G.F.; Dong, X.F.; Ouyang, W. Neuroprotective effects of voluntary exercise and Yisaipu after traumatic brain injury in mice. Sheng Li Xue Bao 2022, 74, 333–352. [Google Scholar]
- Griesbach, G.S.; Gomez-Pinilla, F.; Hovda, D.A. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004, 1016, 154–162. [Google Scholar] [CrossRef]
- Gu, Y.L.; Zhang, L.W.; Ma, N.; Ye, L.L.; Wang, X.; Gao, X. Cognitive improvement of mice induced by exercise prior to traumatic brain injury is associated with cytochrome c oxidase. Neurosci. Lett. 2014, 570, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ou, Y.; Li, J.; Sun, M.; Ge, Q.; Pan, Y.; Cai, Z.; Tan, R.; Wang, W.; An, J.; et al. Voluntary Exercise to Reduce Anxiety Behaviour in Traumatic Brain Injury Shown to Alleviate Inflammatory Brain Response in Mice. Int. J. Mol. Sci. 2023, 24, 6365. [Google Scholar] [CrossRef]
- Itoh, T.; Imano, M.; Nishida, S.; Tsubaki, M.; Hashimoto, S.; Ito, A.; Satou, T. Exercise increases neural stem cell proliferation surrounding the area of damage following rat traumatic brain injury. J. Neural Transm. 2011, 118, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Jacotte-Simancas, A.; Costa-Miserachs, D.; Coll-Andreu, M.; Torras-Garcia, M.; Borlongan, C.V.; Portell-Cortés, I. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis, and neuroprotection after traumatic brain injury in rats. J. Neurotrauma 2015, 32, 739–751. [Google Scholar] [CrossRef]
- Karelina, K.; Schneiderman, K.; Shah, S.; Fitzgerald, J.; Cruz, R.V.; Oliverio, R.; Whitehead, B.; Yang, J.; Weil, Z.M. Moderate Intensity Treadmill Exercise Increases Survival of Newborn Hippocampal Neurons and Improves Neurobehavioral Outcomes after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1858–1869. [Google Scholar] [CrossRef]
- Koo, H.M.; Lee, S.M.; Kim, M.H. Spontaneous Wheel Running Exercise Induces Brain Recovery via Neurotrophin-3 Expression Following Experimental Traumatic Brain Injury in Rats. J. Phys. Ther. Sci. 2013, 25, 1103–1107. [Google Scholar] [CrossRef]
- Lima, F.D.; Oliveira, M.S.; Furian, A.F.; Souza, M.A.; Rambo, L.M.; Ribeiro, L.R.; Silva, L.F.A.; Retamoso, L.T.; Hoffmann, M.S.; Magni, D.V.; et al. Adaptation to oxidative challenge induced by chronic physical exercise prevents Na+,K+-ATPase activity inhibition after traumatic brain injury. Brain Res. 2009, 1279, 147–155. [Google Scholar] [CrossRef]
- Martínez-Drudis, L.; Amorós-Aguilar, L.; Torras-Garcia, M.; Serra-Elias, B.; Costa-Miserachs, D.; Portell-Cortés, I.; Coll-Andreu, M. Delayed voluntary physical exercise restores “when” and “where” object recognition memory after traumatic brain injury. Behav. Brain Res. 2021, 400, 113048. [Google Scholar] [CrossRef]
- Miao, W.; Bao, T.H.; Han, J.H.; Yin, M.; Yan, Y.; Wang, W.W.; Zhu, Y.H. Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz. J. Med. Biol. Res. 2015, 48, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Mychasiuk, R.; Hehar, H.; Ma, I.; Candy, S.; Esser, M.J. Reducing the time interval between concussion and voluntary exercise restores motor impairment, short-term memory, and alterations to gene expression. Eur. J. Neurosci. 2016, 44, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.S.; Stoica, B.A.; Wu, J.; Sabirzhanov, B.; Zhao, Z.; Cabatbat, R.; Loane, D.J.; Faden, A.I. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol. Dis. 2013, 54, 252–263. [Google Scholar] [CrossRef]
- Sánchez-Martín, T.; Costa-Miserachs, D.; Coll-Andreu, M.; Portell-Cortés, I.; García-Brito, S.; Torras-Garcia, M. Treating Traumatic Brain Injury with Exercise: Onset Delay and Previous Training as Key Factors Determining its Efficacy. Neurorehabil. Neural Repair 2024, 38, 715–728. [Google Scholar] [CrossRef]
- Silva, L.F.; Hoffmann, M.S.; Gerbatin Rda, R.; Fiorin Fda, S.; Dobrachinski, F.; Mota, B.C.; Wouters, A.T.; Pavarini, S.P.; Soares, F.A.; Fighera, M.R.; et al. Treadmill exercise protects against pentylenetetrazol-induced seizures and oxidative stress after traumatic brain injury. J. Neurotrauma 2013, 30, 1278–1287. [Google Scholar] [CrossRef]
- Soltani, N.; Soltani, Z.; Khaksari, M.; Ebrahimi, G.; Hajmohammmadi, M.; Iranpour, M. The Changes of Brain Edema and Neurological Outcome, and the Probable Mechanisms in Diffuse Traumatic Brain Injury Induced in Rats with the History of Exercise. Cell. Mol. Neurobiol. 2020, 40, 555–567. [Google Scholar] [CrossRef]
- Szabo, Z.; Ying, Z.; Radak, Z.; Gomez-Pinilla, F. Voluntary exercise may engage proteasome function to benefit the brain after trauma. Brain Res. 2010, 1341, 25–31. [Google Scholar] [CrossRef]
- Tabor, J.; Collins, R.; Debert, C.T.; Shultz, S.R.; Mychasiuk, R. Neuroendocrine whiplash: Slamming the breaks on anabolic-androgenic steroids following repetitive mild traumatic brain injury in rats may worsen outcomes. Front. Neurol. 2019, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Montgomery, M.H.; Gregory, E.J.; Berman, N.E.J. Exercise preconditioning improves traumatic brain injury outcomes. Brain Res. 2015, 1622, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chen, C.C.; Chang, C.P. Effect of stress on the rehabilitation performance of rats with repetitive mild fluid percussion-induced traumatic brain injuries. Cogn. Neurodyn. 2024, 18, 283–297. [Google Scholar] [CrossRef]
- White, B.A.; Ivey, J.T.; Velazquez-Cruz, R.; Oliverio, R.; Whitehead, B.; Pinti, M.; Hollander, J.; Ma, L.; Hu, G.; Weil, Z.M.; et al. Exercise intensity and sex alter neurometabolic, transcriptional, and functional recovery following traumatic brain injury. Exp. Neurol. 2023, 368, 114483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sabirzhanov, B.; Wu, J.; Faden, A.I.; Stoica, B.A. Voluntary Exercise Preconditioning Activates Multiple Antiapoptotic Mechanisms and Improves Neurological Recovery after Experimental Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1347–1360. [Google Scholar] [CrossRef]
- Quan, H.; Koltai, E.; Suzuki, K.; Aguiar, A.S.; Pinho, R.; Boldogh, I.; Berkes, I.; Radak, Z. Exercise, redox system and neurodegenerative diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165778. [Google Scholar] [CrossRef]
- Ooi, S.Z.Y.; Spencer, R.J.; Hodgson, M.; Mehta, S.; Phillips, N.L.; Preest, G.; Manivannan, S.; Wise, M.P.; Galea, J.; Zaben, M. Interleukin-6 as a prognostic biomarker of clinical outcomes after traumatic brain injury: A systematic review. Neurosurg. Rev. 2022, 45, 3035–3054. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Liang, J.; Huang, J.; Chen, N. Exercise suppresses neuroinflammation for alleviating Alzheimer’s disease. J. Neuroinflamm. 2023, 20, 76. [Google Scholar] [CrossRef]
- Andreotti, D.Z.; Silva, J.d.N.; Matumoto, A.M.; Orellana, A.M.; de Mello, P.S.; Kawamoto, E.M. Effects of Physical Exercise on Autophagy and Apoptosis in Aged Brain: Human and Animal Studies. Front. Nutr. 2020, 7, 94. [Google Scholar] [CrossRef]
- Shamsnia, H.S.; Peyrovinasab, A.; Amirlou, D.; Sirouskabiri, S.; Rostamian, F.; Basiri, N.; Shalmani, L.M.; Hashemi, M.; Hushmandi, K.; Abdolghaffari, A.H. BDNF-TrkB Signaling Pathway in Spinal Cord Injury: Insights and Implications. Mol. Neurobiol. 2025, 62, 1904–1944. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H. Neurogenesis and exercise: Past and future directions. Neuromol. Med. 2008, 10, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.D.; Iaccarino, M.A.; Panenka, W.J.; Iverson, G.L.; McCulloch, K.L.; Dams-O’Connor, K.; Reed, N.; McCrea, M.; Cogan, A.M.; Park Graf, M.J.; et al. Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice Guidelines. Arch. Phys. Med. Rehabil. 2020, 101, 382–393. [Google Scholar] [CrossRef]
- Gupte, R.; Brooks, W.; Vukas, R.; Pierce, J.; Harris, J. Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. J. Neurotrauma 2019, 36, 3063–3091. [Google Scholar] [CrossRef]
- Freeman-Jones, E.; Miller, W.H.; Work, L.M.; Fullerton, J.L. Polypathologies and Animal Models of Traumatic Brain Injury. Brain Sci. 2023, 13, 1709. [Google Scholar] [CrossRef]
- Lisi, I.; Moro, F.; Mazzone, E.; Marklund, N.; Pischiutta, F.; Kobeissy, F.; Mao, X.; Corrigan, F.; Helmy, A.; Nasrallah, F.; et al. Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury. Brain 2025, 148, 1062–1080. [Google Scholar] [CrossRef]
- Balakin, E.; Yurku, K.; Fomina, T.; Butkova, T.; Nakhod, V.; Izotov, A.; Kaysheva, A.; Pustovoyt, V. A Systematic Review of Traumatic Brain Injury in Modern Rodent Models: Current Status and Future Prospects. Biology 2024, 13, 813. [Google Scholar] [CrossRef]
- Agoston, D.V.; Vink, R.; Helmy, A.; Risling, M.; Nelson, D.; Prins, M. How to Translate Time: The Temporal Aspects of Rodent and Human Pathobiological Processes in Traumatic Brain Injury. J. Neurotrauma 2019, 36, 1724–1737. [Google Scholar] [CrossRef]
- Bagnato, S.; Boccagni, C. Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review. Brain Sci. 2023, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Zare, N.; Bishop, D.J.; Levinger, I.; Febbraio, M.A.; Broatch, J.R. Exercise intensity matters: A review on evaluating the effects of aerobic exercise intensity on muscle-derived neuroprotective myokines. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2025, 11, e70056. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17–52. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Antonioni, A.; Baroni, A.; Bonsangue, V.; Lavezzi, S.; Koch, G.; Tisato, V.; Ziliotto, N.; Basaglia, N.; Secchiero, P.; et al. Anti-Inflammatory and Cortical Responses after Transcranial Direct Current Stimulation in Disorders of Consciousness: An Exploratory Study. J. Clin. Med. 2024, 13, 108. [Google Scholar] [CrossRef]
- Scheffer, D.d.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]


| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adkins 2015 [41] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Amorós-Aguilar 2020 [42] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Barrett 2025 [43] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Chen 2013 [44] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Chio 2017 [14] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Chou 2018 [45] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Combs 2016 [46] | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Crane 2012 [47] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| de Castro 2017 [48] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Ferguson 2020 [49] | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Gan 2022 [50] | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Griesbach 2004 [51] | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 |
| Gu 2014 [52] | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| Hu 2023 [53] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Itoh 2011a [54] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 5 |
| Itoh 2011b [5] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 5 |
| Jacotte-Simancas 2015 [55] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Karelina 2021 [56] | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Koo 2013 [57] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Lima 2009 [58] | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Martínez-Drudis 2021 [59] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Miao 2015 [60] | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| Mota 2012 [6] | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 |
| Mychasiuk 2016 [61] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Piao 2013 [62] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 6 |
| Rafie 2024 [7] | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Sánchez-Martín 2024 [63] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| Silva 2013 [64] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Soltani 2020 [65] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Szabo 2010 [66] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 5 |
| Tabor 2019 [67] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 7 |
| Taylor 2015 [68] | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Wang 2024 [69] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| White 2023 [70] | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Zhao 2015 [71] | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Study | Study Characteristics | Outcome Measures | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author-Year | TBI Model | Exercise Training Protocol | Oxidative Stress | Inflammatory Pathway | Apoptosis | Mitochondrial Function | Neurotrophic Factors | Neurogenesis | Motor Outcomes |
| Adkins 2015 [41] | TBI: CCI n: 9 vs. 9 A: 12 wks S/Sp: Male rats Wt: 250–350 g | WR t: 6 h/day T: 28 d | - | - | - | - | - | - | Reaching activity ↑, fine motor activity ↔ |
| Amorós-Aguilar 2020 [42] | TBI: CCI n: 13 vs. 17 A: 7 wks S/Sp: Male rats Wt: 230 g | WR T: 21 d | - | IBA1 ↔ | - | - | - | NeuN+ ↑ | |
| Barrett 2025 [43] | TBI: CCI n: 8 vs. 12 A: 10 wks S/Sp: Male mice | WR T: 56 d | mRNA Tnf ↓, Itgam ↔, Gfap ↔, and Il1b ↓. GFAP ↔, STAT3 ↓, IBA1 ↓ | ASC↔ | - | - | - | - | |
| Chen 2013 [44] | TBI: CHI n: 6–12 vs. 6–12 A: 7 wks S/Sp: mice | TR t: 1 h/day T: 14 d v: 9–13.5 m/min | - | IBA1 ↓ | - | - | - | NeuN+ ↑ | NSS ↓ |
| Chio 2017 [14] | TBI: FPI n: 8/8 S/Sp: Male rats Wt: 310 g | TR T: 21 d f: 5 d/wk | - | IL-6 ↑, NF-κB binding at IL-6 Promotor ↑ | HSP70 ↑, Apoptosis ↓ | - | - | Syn-I ↑ | - |
| Chou 2018 [45] | TBI: FPI n: 10 vs. 10 S/Sp: Male rats Wt: 265 g | WR T: 24 d t: 30–60 min, v: 20–30 m/min | - | - | HSP 20 ↑ | - | BDNF ↑, TrkB ↑ | - | - |
| Combs 2016 [46] | TBI: CCI n: 22 vs. 25 A: 14 wks S/Sp: Male rats | WR t: 6 h/day + reach T: 28 d | - | - | - | - | - | - | Reach accuracy ↑, motor movement ↑, wrist motor function ↑ |
| Crane 2012 [47] | TBI: PCI n: 6 vs. 8 A: 7 wks S/Sp: Male rats Wt: 290 g | WR T: 19 d | - | GFAP ↔, IBA ↔ | DAPI ↔ | - | - | - | - |
| de Castro 2017 [48] | TBI: FPI n: 6 vs. 6 S/Sp: Male rats Wt: 250–350 g | Swimming t: 60 min/d T: 40 d f: 5 d/wk | Na+/K+-ATPase activity ↑ | TNF-α ↓, IL-6 ↓, MPO activity ↓ | - | - | - | - | - |
| Ferguson 2020 [49] | TBI: CHI n: 7 vs. 7 A: 5 wks S/Sp: Male rats Wt: 100–140 g | WR T: 18 d | - | - | - | PGC-1α ↑ | BDNF ↑ | - | - |
| Gan 2022 [50] | TBI: FPI n: 13 vs. 12 A: 5 wks S/Sp: Male mice | WR T: 36 d f: 6 d/wk | - | GFAP ↓ | - | - | - | - | Swing velocity ↑ |
| Griesbach 2004 [51] | TBI: FPI n: 4 vs. 4 S/Sp: Male rats Wt: 250–300 g | WR T: 7 d | - | - | - | - | p-CREB ↓, CREB ↓, PKC ↓, CAMKII ↓, p-MAPKI ↓, MAPKII ↓ | T and p-Syn I ↓, | - |
| Gu 2014 [52] | TBI: CCI n: 13 vs. 13 A: 16 wks S/Sp: Male mice | WR T: 21 d | - | GFAP ↓ | - | - | - | NeuN+ ↑, GAP43 ↑ | - |
| Hu 2023 [53] | TBI: FFFI n: 12 vs. 15 A: 24 wks S/Sp: Male mice | WR T: 7 d | - | mRNA Il1b ↓, Il12 ↓, Ifng ↓, Ccl2 ↓, Il10 ↑, Tgfb ↑. NLRP3 ↓, IL-1β ↓, IL-18 ↓, IBA1 ↓, CD68 ↓, iNOS ↓, CD16 ↓, Ym-1 ↓, Arg-1 ↓, CD206 ↓ | - | - | - | - | NSS ↓ |
| Itoh 2011a [54] | TBI: CCI n: 36 vs. 36 A: 10 wks S/Sp: Male rats Wt: 200–250 g | TR t: 30 min/d T: 7 d v: 22 m/min | - | - | - | - | - | NSC ↑, Ki-67 ↑, neurospheres ↑ | - |
| Itoh 2011b [5] | TBI: PCI n: 26 vs. 26 A: 10 wks S/Sp: Male rats Wt: 200–250 g | TR t: 30 min/d T: 7 d v: 22 m/min | - | GFAP ↓ | ssDNA ↓ | - | - | NeuN+ ↑ | cerebral function ↑ |
| Jacotte-Simancas 2015 [55] | TBI: CCI n: 9 vs. 11 A: 7 wks S/Sp: Male rats Wt: 250 g | WR T: 20 d | - | - | - | - | - | NeuN+ ↑ | - |
| Karelina 2021 [56] | TBI: CCI n: 15 vs. 15 A: 4–6 wks S/Sp: Male mice | TR t:10–30 min/d T: 13 d v: 6–15 m/min | - | IBA1 ↓ | - | - | - | - | - |
| Koo 2013 [57] | TBI: CCI n: 10 vs. 10 S/Sp: Male rats Wt: 250–300 g | WR T: 21 d t: 15 min/d | - | - | - | - | NT-3 ↑ | - | - |
| Lima 2009 [58] | TBI: FPI n: 8 vs. 8 A: 13 wks S/Sp: Male rats Wt: 220–320 g | Swimming T: 30 d t: 60 min/d f: 5 d/wk | Carbonyl ↓, TBARS ↓, Na+/K+-ATPase ↑, and its α1 subunit activity ↑ | - | - | - | - | - | - |
| Martínez-Drudis 2021 [59] | TBI: CCI n: 9 vs. 10 A: 7 wks S/Sp: Male rats | WR T: 25 d | - | - | - | - | BDNF ↔ | - | - |
| Miao 2015 [60] | TBI: CCI n: 30 vs. 30 A: 16 wks S/Sp: Male mice | WR T: 21 d | let-7c ↑ | - | miR-21 ↓, miR92a ↓, miR-874 ↓ | - | - | miR-138 ↑, miR124 ↑ | - |
| Mota 2012 [6] | TBI: FPI n: 8–9 vs. 8–9 A: 13 wks S/Sp: Male rats Wt: 220–260 g | TR T: 28 d | Na+/K+-ATPase activity ↑ | IL-1β ↓, TNF-α ↓, IL-6 ↔, IL-10 ↑, MPO activity ↓ | - | - | - | - | Motor function ↑ |
| Mychasiuk 2016 [61] | TBI: LI n: 8 vs. 11 S/Sp: Male/Female rats | WR T: 7 d | - | - | mRNA Tert ↑, TL ↑ | BDNF ↑, Pgc1-α ↑, Igf-1 ↑ | mRNA Dnmt1 ↑ | - | |
| Piao 2013 [62] | TBI: CCI n: 15 vs. 15 A: 10 wks S/Sp: Male mice | WR T: 63 d | gp91^phox and p22^phox ↓ | IL-1β ↓, IL-6 ↑, IL-10 ↑, C1qB ↓, Gelactin-3 ↓ | - | - | - | - | - |
| Rafie 2024 [7] | TBI: WDM n: 8 vs. 8 S/Sp: Male rats Wt: 250–300 g | TR t: 30 min/d T: 40 d f: 5 d/wk | - | - | Apoptosis ↓ | - | - | - | Motor coordination & function ↑ |
| Sánchez-Martín 2024 [63] | TBI: CCI n: 9 vs. 12 A: 6 wks S/Sp: Male rats Wt: 250 g | WR T: 9 d | - | - | - | - | - | NeuN+ ↑ | - |
| Silva 2013 [64] | TBI: FPI n: 9 vs. 11 S/Sp: Male rats Wt: 250–300 g | TR; T: 20 d f: 4–5 d/wk | GSH ↑, GSH/GSSG ↑, carbonyl ↓, TBARS ↓, SOD ↑, Na/K ATPase ↑ | - | - | - | - | Neuron loss ↔ | - |
| Soltani 2020 [65] | TBI: MIA n: 12 S/Sp: Male rats Wt: 180–210 g | TR t: 30 min/d T: 40 d f: 5 d/wk v: 20–25 m/min | MDA ↓, Carbonyl ↓ | IL-1β ↓ | - | - | - | - | VCS ↓ |
| Szabo 2010 [66] | TBI: FPI n: 5 vs. 4 S/Sp: Male rats Wt: 250–300 g | WR T: 14 d | Carbonyl ↓ | - | - | - | - | Syn-I ↑, Corticosterone-like Activity ↓, Zif268 ↓, 20S proteasome ↓ | - |
| Tabor 2019 [67] | TBI: LI n: 8 vs. 15 A: 3 wks S/Sp: Male rats | WR T: 14 d | - | mRNA Aif1 ↓ | - | - | mRNA Bdnf ↑ | - | - |
| Taylor 2015 [68] | TBI: CCI n: 30 vs. 30 A: 20 wks S/Sp: Male mice | WR T: 42 d | - | - | - | - | - | mRNA Vegfa ↑, Epo ↑. VEGF-A ↑ EPO ↑ | - |
| Wang 2024 [69] | TBI: FPI n: 8 vs. 8 S/Sp: Male rats Wt: 250–350 g | WR t: 30 min/d T: 21 d | - | - | - | - | - | - | mNSS ↓, |
| White 2023 [70] | TBI: CCI n: 5–7 vs. 5–7 A: 5 wks S/Sp: Male/Female mice | TR t: 10–30 min/d T: 13 d v: 6 m/min | GSH/GSSG ↑ | - | - | Mito. Respiration ↑, ATP ↑ | - | - | - |
| Zhao 2015 [71] | TBI: CCI n: 15 vs. 17 S/Sp: Male mice A: 10 wks | WR T: 28 d | - | IBA-1 ↓ | mRNA Bid ↓ and Bbc3 ↓. Caspase activation ↓, Cyt c & AIF translocation ↓, mRNA Hspa1a ↑ | mRNA Bdnf ↑, mRNA Creb1 ↑ | Neuronal Density ↑ | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Yousaf, F.; Kao, S.; Ishaq, S.; Lee, S.-D. Cortical Neuroprotective Mechanisms of Exercise Training in Post-Traumatic Brain Injury: A Systematic Review. Int. J. Mol. Sci. 2026, 27, 52. https://doi.org/10.3390/ijms27010052
Yousaf F, Kao S, Ishaq S, Lee S-D. Cortical Neuroprotective Mechanisms of Exercise Training in Post-Traumatic Brain Injury: A Systematic Review. International Journal of Molecular Sciences. 2026; 27(1):52. https://doi.org/10.3390/ijms27010052
Chicago/Turabian StyleYousaf, Farhan, Sean Kao, Shahid Ishaq, and Shin-Da Lee. 2026. "Cortical Neuroprotective Mechanisms of Exercise Training in Post-Traumatic Brain Injury: A Systematic Review" International Journal of Molecular Sciences 27, no. 1: 52. https://doi.org/10.3390/ijms27010052
APA StyleYousaf, F., Kao, S., Ishaq, S., & Lee, S.-D. (2026). Cortical Neuroprotective Mechanisms of Exercise Training in Post-Traumatic Brain Injury: A Systematic Review. International Journal of Molecular Sciences, 27(1), 52. https://doi.org/10.3390/ijms27010052









