Recent Advances in Photoelectrochemical Nitrate Reduction to Ammonia
Abstract
1. Introduction
2. Photocathodes for PEC Nitrate Reduction to NH3
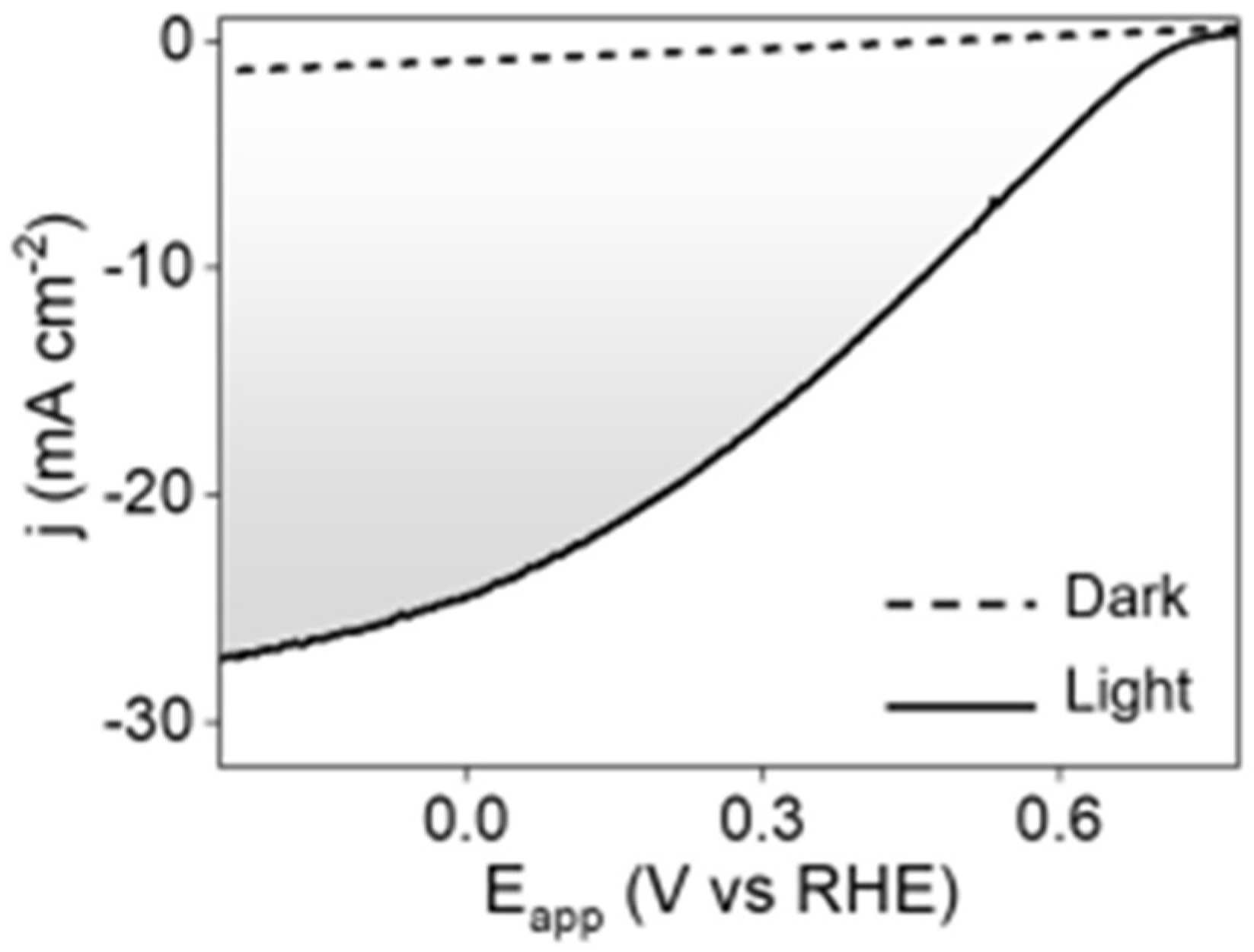
2.1. Antimony-Based Photocathodes
2.2. Silicon
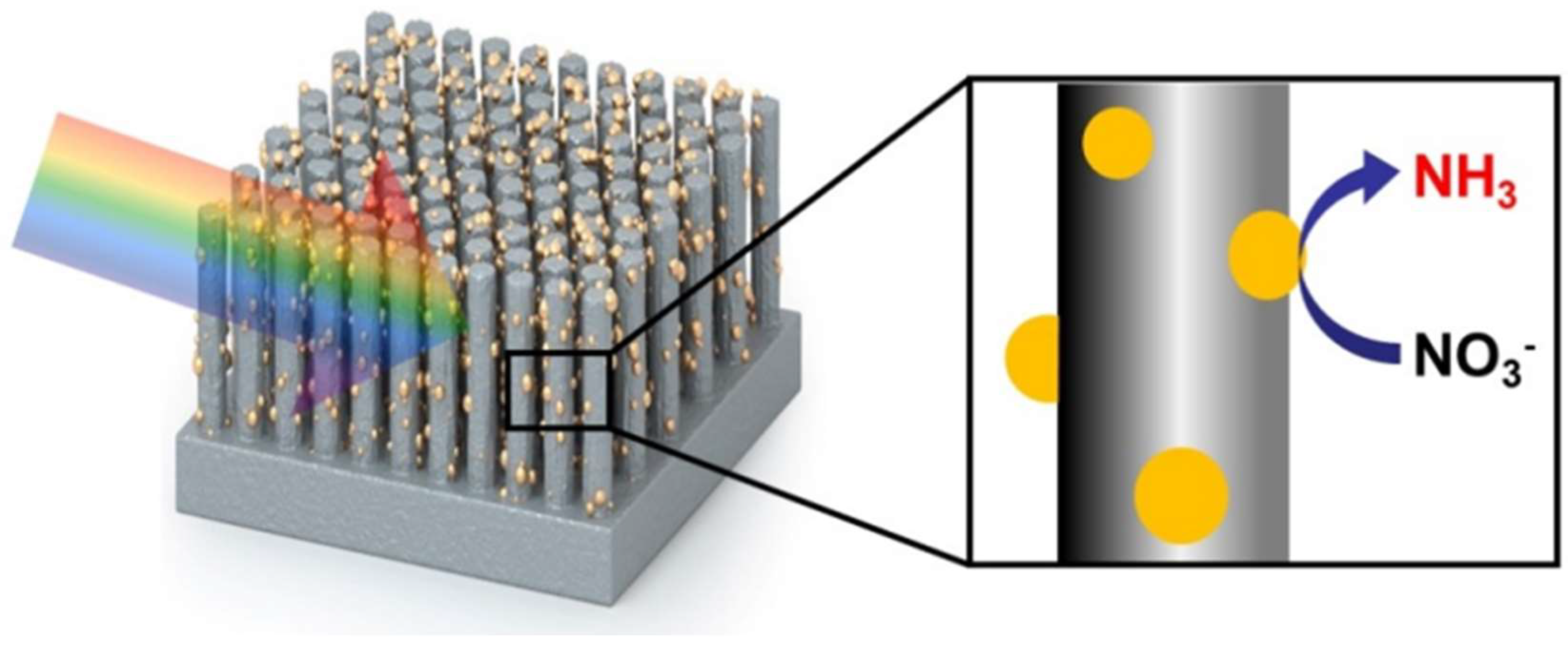
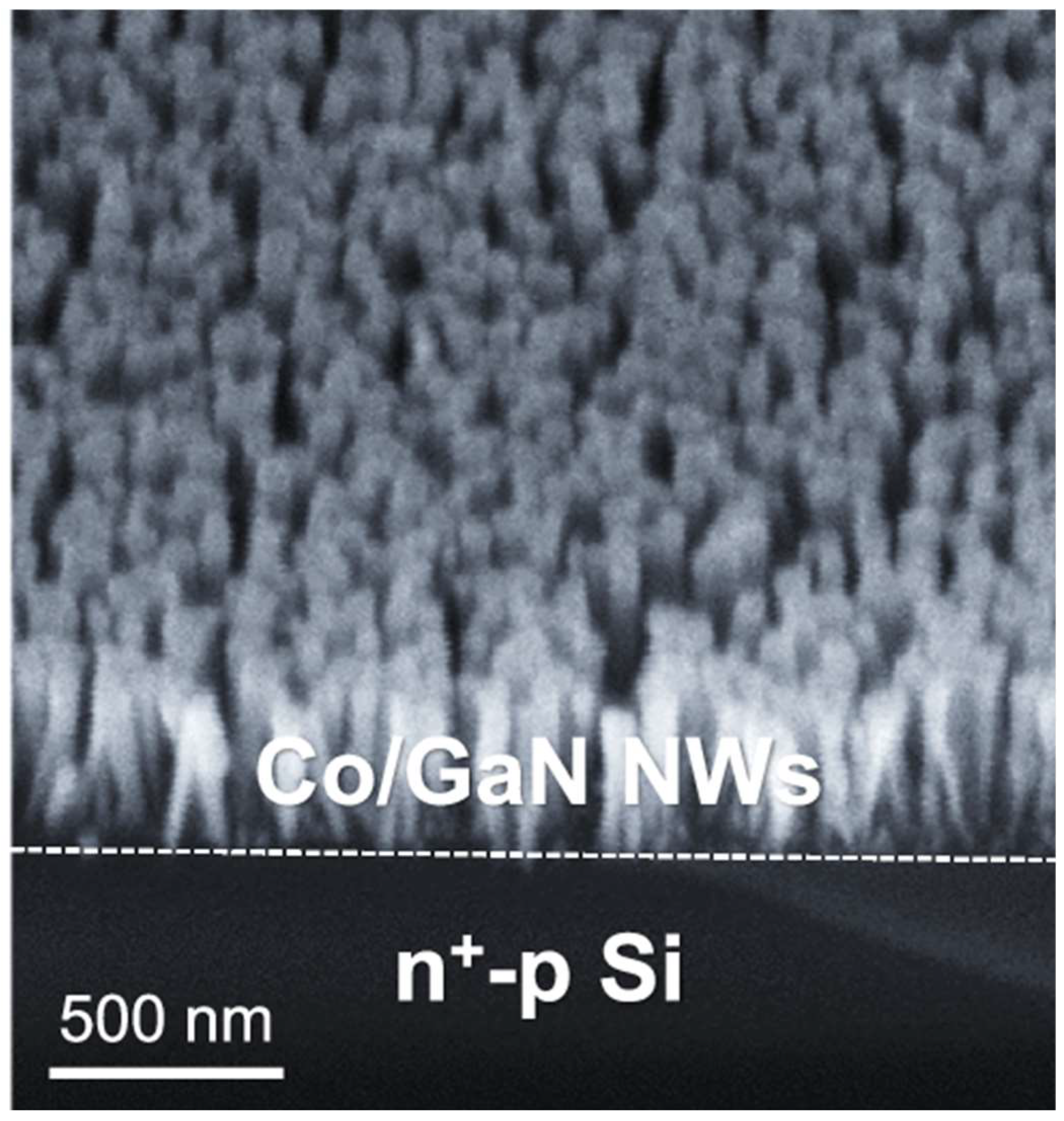
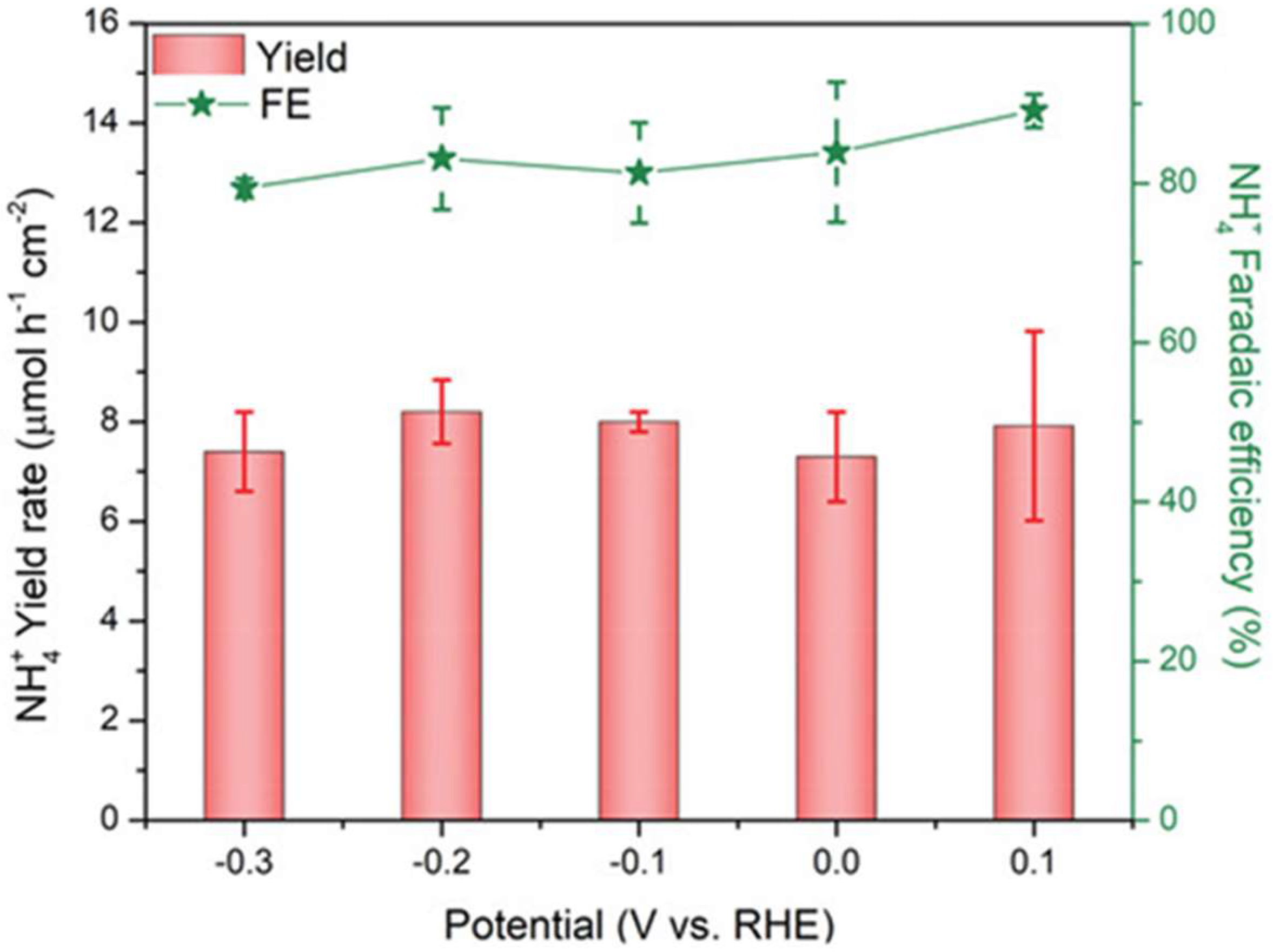
2.3. Gallium Nitride
2.4. Oxides
2.5. Cu2ZnSnS4
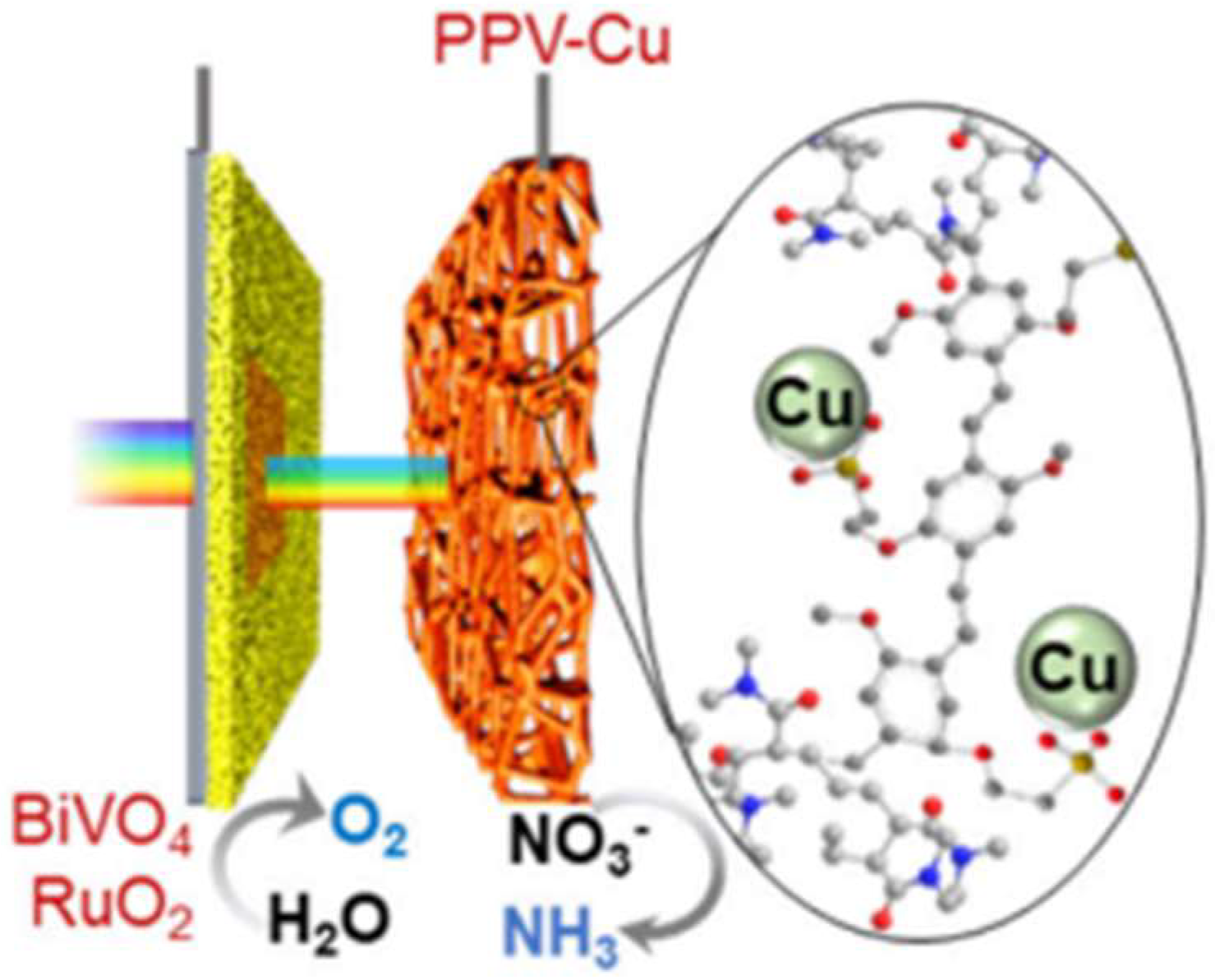
2.6. Organic Semiconductor Photocathodes
2.7. Organic–Inorganic Hybrid Perovskites
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hollevoet, L.; De Ras, M.; Roeffaers, M.; Hofkens, J.; Martens, J.A. Energy-Efficient Ammonia Production from Air and Water Using Electrocatalysts with Limited Faradaic Efficiency. ACS Energy Lett. 2020, 5, 1124–1127. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Li, T.; Chen, F.; Yang, R.; Yu, Y.; Zhang, B. Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism. Nat. Catal. 2023, 6, 402–414. [Google Scholar] [CrossRef]
- van Langevelde, P.H.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Nitrate Reduction for Sustainable Ammonia Production. Joule 2021, 5, 290–294. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Adv. Energy Mater. 2018, 8, 1800369. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, G.; Chen, G.-F.; Zhang, H.; Zhang, S.; Wang, H. Comprehensive Understanding of the Thriving Ambient Electrochemical Nitrogen Reduction Reaction. Adv. Mater. 2021, 33, 2007650. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Huang, J.; Chen, J.; Kong, Y.; Yang, B.; Li, Z.; Lei, L.; Chai, G.; Wen, Z.; et al. Boosting Electroreduction Kinetics of Nitrogen to Ammonia via Tuning Electron Distribution of Single-Atomic Iron Sites. Angew. Chem. Int. Ed. 2021, 60, 9078–9085. [Google Scholar] [CrossRef]
- Wang, S.; Ichihara, F.; Pang, H.; Chen, H.; Ye, J. Nitrogen Fixation Reaction Derived from Nanostructured Catalytic Materials. Adv. Funct. Mater. 2018, 28, 1803309. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Liu, H. Ammonia synthesis catalyst 100 years: Practice, enlightenment and challenge. Chin. J. Catal. 2014, 35, 1619–1640. [Google Scholar] [CrossRef]
- Greenlee, L.F. Recycling fertilizer. Nat. Energy 2020, 5, 557–558. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An Electrochemical Haber-Bosch Process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Capdevila-Cortada, M. Electrifying the Haber–Bosch. Nat. Catal. 2019, 2, 1055. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Jaramillo, T.F.; Nørskov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef]
- van der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Bossola, F.; Tuci, G.; Sangiorgi, N.; Sanson, A.; Dal Santo, V.; Psaro, R.; Giambastiani, G. Iron-group single-atom catalysts for ammonia synthesis and decomposition. Rend. Fis. Acc. Lincei 2025, 36, 659–676. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Faria, J.L.; Silva, C.G. Photocatalytic nitrate reduction over Pd–Cu/TiO2. Chem. Eng. J. 2014, 251, 123–130. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, M.; Yu, Y.; Zhang, B. Nitrate electroreduction: Mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 2021, 50, 6720–6733. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Vasileff, A.; Qiao, S.-Z. The Hydrogen Evolution Reaction in Alkaline Solution: From Theory, Single Crystal Models, to Practical Electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 7568–7579. [Google Scholar] [CrossRef]
- Gao, W.; Xie, K.; Xie, J.; Wang, X.; Zhang, H.; Chen, S.; Wang, H.; Li, Z.; Li, C. Alloying of Cu with Ru Enabling the Relay Catalysis for Reduction of Nitrate to Ammonia. Adv. Mater. 2023, 35, 2202952. [Google Scholar] [CrossRef]
- Kanter, D.R.; Chodos, O.; Nordland, O.; Rutigliano, M.; Winiwarter, W. Gaps and opportunities in nitrogen pollution policies around the world. Nat. Sustain. 2020, 3, 956–963. [Google Scholar] [CrossRef]
- Kou, Z.; Shi, D.; Yang, B.; Li, Z.; Zhang, Q.; Lu, J.; Zhang, T.; Lei, L.; Li, Y.; Dai, L.; et al. Efficient green synthesis of ammonia: From mechanistic understanding to reactor design for potential production. Chem. Soc. Rev. 2025, 54, 10796–10844. [Google Scholar] [CrossRef]
- Han, C.; Wang, K. Recent Advances in Photoelectrochemical Synthesis of Nitrogen-Containing Solar Fuels and Chemicals. Energy Fuels 2025, 39, 16065–16077. [Google Scholar] [CrossRef]
- Sendeku, M.G.; Shifa, T.A.; Dajan, F.T.; Ibrahim, K.B.; Wu, B.; Yang, Y.; Moretti, E.; Vomiero, A.; Wang, F. Frontiers in Photoelectrochemical Catalysis: A Focus on Valuable Product Synthesis. Adv. Mater. 2024, 36, 2308101. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Zheng, J.; Lyu, Y.; Qiao, M.; Wang, R.; Zhou, Y.; Li, H.; Chen, C.; Li, Y.; Zhou, H.; Jiang, S.P.; et al. Photoelectrochemical Synthesis of Ammonia on the Aerophilic-Hydrophilic Heterostructure with 37.8% Efficiency. Chem 2019, 5, 617–633. [Google Scholar] [CrossRef]
- Qing, G.; Ghazfar, R.; Jackowski, S.T.; Habibzadeh, F.; Ashtiani, M.M.; Chen, C.-P.; Smith, M.R., III; Hamann, T.W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Jia, R.; Zhang, C.; Zhang, B. Electrochemical synthesis of nitric acid from air and ammonia through waste utilization. Natl. Sci. Rev. 2019, 6, 730–738. [Google Scholar] [CrossRef]
- Duca, M.; Koper, M.T.M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 2012, 5, 9726–9742. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim. Acta 2008, 53, 5977–5984. [Google Scholar] [CrossRef]
- Butcher, D.P.; Gewirth, A.A. Nitrate reduction pathways on Cu single crystal surfaces: Effect of oxide and Cl−. Nano Energy 2016, 29, 457–465. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications. Appl. Catal. B Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the Nitrogen Cycle by Electrochemical Reduction of Nitrate: Progress and Prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Zhang, J.; Deng, B.; Cao, Z.; Wang, Z.; Wei, G.; Zhang, Q.; Jia, R.; Xiang, P.; et al. Critical review in electrocatalytic nitrate reduction to ammonia towards a sustainable nitrogen utilization. Chem. Eng. J. 2024, 483, 148952. [Google Scholar] [CrossRef]
- Dong, W.J.; Mi, Z. Recent advances in photoelectrochemical ammonia synthesis. Nano Futures 2025, 9, 032002. [Google Scholar] [CrossRef]
- Tan, J.; Kang, B.; Kim, K.; Kang, D.; Lee, H.; Ma, S.; Jang, G.; Lee, H.; Moon, J. Hydrogel protection strategy to stabilize water-splitting photoelectrodes. Nat. Energy 2022, 7, 537–547. [Google Scholar] [CrossRef]
- Yang, W.; Kim, J.H.; Hutter, O.S.; Phillips, L.J.; Tan, J.; Park, J.; Lee, H.; Major, J.D.; Lee, J.S.; Moon, J. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat. Commun. 2020, 11, 861. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.-B.; Li, K.; Chen, C.; Deng, H.-X.; Gao, L.; Zhao, Y.; Jiang, F.; Li, L.; Huang, F.; et al. Stable 6%-efficient Sb2Se3 solar cells with a ZnO buffer layer. Nat. Energy 2017, 2, 17046. [Google Scholar] [CrossRef]
- Qin, G.; Liu, B.; Liu, Y.; Xiao, Y.; Liu, F.; Wang, L. In Situ Constructing Polymer Conductors with Aromatic Side Chains Functionalized Sb2Se3 Complex for Enhanced Potassium Motion. Adv. Energy Mater. 2023, 13, 2300414. [Google Scholar] [CrossRef]
- Kim, J.; Yang, W.; Oh, Y.; Lee, H.; Lee, S.; Shin, H.; Kim, J.; Moon, J. Self-oriented Sb2Se3 nanoneedle photocathodes for water splitting obtained by a simple spin-coating method. J. Mater. Chem. A 2017, 5, 2180–2187. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Ishaq, M.; Chen, Z.; Tang, R.; Zheng, Z.; Su, Z.; Fan, P.; Zhang, X.; Chen, S. Heterojunction interface engineering enabling high onset potential in Sb2Se3/CdS photocathodes for efficient solar hydrogen production. Chem. Eng. J. 2022, 431, 133359. [Google Scholar] [CrossRef]
- Wang, Z.; Bae, S.; Baljozović, M.; Adams, P.; Yong, D.; Service, E.; Moehl, T.; Niu, W.; Tilley, S.D. One-Step Hydrothermal Synthesis of Sn-Doped Sb2Se3 for Solar Hydrogen Production. ACS Catal. 2024, 14, 9877–9886. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Gao, R.-T.; Nguyen, N.T.; Wang, L. Enhanced Charge Carrier Dynamics on Sb2Se3 Photocathodes for Efficient Photoelectrochemical Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202317414. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Zhu, Y.; Cai, X.; Xiao, F.; Ma, T.; Yang, J.; Shen, G.; Ke, A.; Lu, Y.; et al. A Codoping Strategy for Efficient Planar Heterojunction Sb2S3 Solar Cells. Adv. Energy Mater. 2022, 12, 2202897. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, N.; Zhang, F.; Wang, K.; Fu, Y.; Ye, Z.; Jiang, J.; Zhu, L. A High-Performance Sb2S3–Based Photocathode with rGO and TiO2/Pt for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2025, 8, 9175–9187. [Google Scholar] [CrossRef]
- Feng, K.; Zhao, B.; Fan, Z.; Li, R.; Zhang, B.; Li, Y. Solar-driven bipolar hydrogen production using a large-grain Sb2S3 photocathode coupled with a Cu2O anode. Chem. Eng. J. 2025, 521, 166622. [Google Scholar] [CrossRef]
- Kondrotas, R.; Chen, C.; Tang, J. Sb2S3 Solar Cells. Joule 2018, 2, 857–878. [Google Scholar] [CrossRef]
- Yang, M.; Fan, Z.; Du, J.; Li, R.; Liu, D.; Zhang, B.; Feng, K.; Feng, C.; Li, Y. Tailoring the Crystallographic Orientation of a Sb2S3 Thin Film for Efficient Photoelectrochemical Water Reduction. ACS Catal. 2022, 12, 8175–8184. [Google Scholar] [CrossRef]
- Kumar, M.; Ghosh, C.C.; Meena, B.; Ma, T.; Subrahmanyam, C. Plasmonic Au nanoparticle sandwiched CuBi2O4/Sb2S3 photocathode with multi-mediated electron transfer for efficient solar water splitting. Sustain. Energy Fuels 2022, 6, 3961–3974. [Google Scholar] [CrossRef]
- Ren, S.; Gao, R.-T.; Yu, J.; Yang, Y.; Liu, X.; Wu, L.; Wang, L. Enhanced Charge-Carrier Dynamics and Efficient Photoelectrochemical Nitrate-to-Ammonia Conversion on Antimony Sulfide-Based Photocathodes. Angew. Chem. Int. Ed. 2024, 63, e202409693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Andrei, V.; Ucoski, G.M.; Pornrungroj, C.; Uswachoke, C.; Wang, Q.; Achilleos, D.S.; Kasap, H.; Sokol, K.P.; Jagt, R.A.; Lu, H.; et al. Floating perovskite-BiVO4 devices for scalable solar fuel production. Nature 2022, 608, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lee, B.G.; Lin, C.; Liu, T.-K.; Wang, Z.; Miao, J.; Oh, S.H.; Kim, K.C.; Zhang, K.; Park, J.H. Solar-driven highly selective conversion of glycerol to dihydroxyacetone using surface atom engineered BiVO4 photoanodes. Nat. Commun. 2024, 15, 5475. [Google Scholar] [CrossRef]
- Luo, L.; Chen, W.; Xu, S.-M.; Yang, J.; Li, M.; Zhou, H.; Xu, M.; Shao, M.; Kong, X.; Li, Z.; et al. Selective Photoelectrocatalytic Glycerol Oxidation to Dihydroxyacetone via Enhanced Middle Hydroxyl Adsorption over a Bi2O3-Incorporated Catalyst. J. Am. Chem. Soc. 2022, 144, 7720–7730. [Google Scholar] [CrossRef]
- Kim, S.; Oh, D.; Jang, J.-W. Unassisted Photoelectrochemical H2O2 Production with In Situ Glycerol Valorization Using α-Fe2O3. Nano Lett. 2024, 24, 5146–5153. [Google Scholar] [CrossRef]
- Guo, X.; Gao, R.-T.; Ren, S.; Nguyen, N.T.; Chen, H.; Wu, L.; Wang, L. Direct ammonia and dihydroxyacetone production in an unbiased photoelectrochemical cell. Nat. Commun. 2025, 16, 6220. [Google Scholar] [CrossRef]
- Su, K.; Ren, S.; Gao, R.-T.; Bai, G.-E.; Wu, L.; Wang, L. Bias-Free Solar-Driven Ammonia Coupled to C3-Dihydroxyacetone Production through Photoelectrochemistry. Angew. Chem. Int. Ed. 2025, 64, e202422443. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, T.; Gong, J. Single-crystal silicon-based electrodes for unbiased solar water splitting: Current status and prospects. Chem. Soc. Rev. 2019, 48, 2158–2181. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Navid, I.A.; Xiao, Y.; Lim, J.W.; Lee, J.-L.; Mi, Z. CuS-Decorated GaN Nanowires on Silicon Photocathodes for Converting CO2 Mixture Gas to HCOOH. J. Am. Chem. Soc. 2021, 143, 10099–10107. [Google Scholar] [CrossRef]
- Oh, I.; Kye, J.; Hwang, S. Enhanced Photoelectrochemical Hydrogen Production from Silicon Nanowire Array Photocathode. Nano Lett. 2012, 12, 298–302. [Google Scholar] [CrossRef]
- Lu, L.; Li, Z.; Chen, X.; Wang, H.; Dai, S.; Pan, X.; Ren, Z.J.; Gu, J. Spontaneous Solar Syngas Production from CO2 Driven by Energetically Favorable Wastewater Microbial Anodes. Joule 2020, 4, 2149–2161. [Google Scholar] [CrossRef]
- Zhang, H.; Li, A.; Wang, Z.; Ma, W.; Li, D.; Zong, X.; Li, C. Decorating mesoporous silicon with amorphous metal–phosphorous-derived nanocatalysts towards enhanced photoelectrochemical water reduction. J. Mater. Chem. A 2016, 4, 14960–14967. [Google Scholar] [CrossRef]
- Xu, M.; Xu, F.; Zhu, K.; Xu, X.; Deng, P.; Wu, W.; Ye, W.; Sun, Z.; Gao, P. Atomic layer deposition technique refining oxygen vacancies in TiO2 passivation layer for photoelectrochemical ammonia synthesis. Compos. Commun. 2022, 29, 101037. [Google Scholar] [CrossRef]
- Kim, H.E.; Kim, J.; Ra, E.C.; Zhang, H.; Jang, Y.J.; Lee, J.S. Photoelectrochemical Nitrate Reduction to Ammonia on Ordered Silicon Nanowire Array Photocathodes. Angew. Chem. Int. Ed. 2022, 61, e202204117. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Peng, S.; Qiao, L.; Bai, H.; An, K.; Liu, C.; Chen, M.; Lo, K.H.; Ng, K.W.; Peng, S.; et al. Rational design of cocatalyst for highly improved ammonia production from photoelectrochemical nitrate reduction. Appl. Catal. B Environ. Energy 2024, 351, 123980. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Dai, H.; He, D.; Ke, Z.; Xiao, X. Photoelectrochemical nitrate denitrification towards acidic ammonia synthesis on copper-decorated black silicon. Energy Environ. Sci. 2024, 17, 9233–9243. [Google Scholar] [CrossRef]
- Jin, W.; Go, H.; Jeong, J.; Park, J.; Tayyebi, A.; Yu, J.M.; Kim, S.; Choi, K.; Jang, J.-W.; Seo, K. Nickel Hydroxide Catalyzed Bias-free Photoelectrochemical NH3 Production via Nitrate Reduction. Adv. Mater. 2025, 37, 2506567. [Google Scholar] [CrossRef]
- Varadhan, P.; Fu, H.-C.; Kao, Y.-C.; Horng, R.-H.; He, J.-H. An efficient and stable photoelectrochemical system with 9% solar-to-hydrogen conversion efficiency via InGaP/GaAs double junction. Nat. Commun. 2019, 10, 5282. [Google Scholar] [CrossRef]
- Young, J.L.; Steiner, M.A.; Döscher, H.; France, R.M.; Turner, J.A.; Deutsch, T.G. Direct solar-to-hydrogen conversion via inverted metamorphic multi-junction semiconductor architectures. Nat. Energy 2017, 2, 17028. [Google Scholar] [CrossRef]
- Liu, B.; Feng, S.; Yang, L.; Li, C.; Luo, Z.; Wang, T.; Gong, J. Bifacial passivation of n-silicon metal–insulator–semiconductor photoelectrodes for efficient oxygen and hydrogen evolution reactions. Energy Environ. Sci. 2020, 13, 221–228. [Google Scholar] [CrossRef]
- Han, C.; Li, C.; Yuwono, J.A.; Liu, Z.; Sun, K.; Wang, K.; He, G.; Huang, J.; Kumar, P.V.; Vongsvivut, J.; et al. Nanostructured hybrid catalysts empower the artificial leaf for solar-driven ammonia production from nitrate. Energy Environ. Sci. 2024, 17, 5653–5665. [Google Scholar] [CrossRef]
- Kibria, M.G.; Qiao, R.; Yang, W.; Boukahil, I.; Kong, X.; Chowdhury, F.A.; Trudeau, M.L.; Ji, W.; Guo, H.; Himpsel, F.J.; et al. Atomic-Scale Origin of Long-Term Stability and High Performance of p-GaN Nanowire Arrays for Photocatalytic Overall Pure Water Splitting. Adv. Mater. 2016, 28, 8388–8397. [Google Scholar] [CrossRef]
- Dong, W.J.; Xiao, Y.; Yang, K.R.; Ye, Z.; Zhou, P.; Navid, I.A.; Batista, V.S.; Mi, Z. Pt nanoclusters on GaN nanowires for solar-asssisted seawater hydrogen evolution. Nat. Commun. 2023, 14, 179. [Google Scholar] [CrossRef]
- Xiao, Y.; Vanka, S.; Pham, T.A.; Dong, W.J.; Sun, Y.; Liu, X.; Navid, I.A.; Varley, J.B.; Hajibabaei, H.; Hamann, T.W.; et al. Crystallographic Effects of GaN Nanostructures in Photoelectrochemical Reaction. Nano Lett. 2022, 22, 2236–2243. [Google Scholar] [CrossRef]
- Dong, W.J.; Menzel, J.P.; Ye, Z.; Long, Z.; Navid, I.A.; Batista, V.S.; Mi, Z. Synergistic Metal-Support Interactions in Au/GaN Catalysts for Photoelectrochemical Nitrate Reduction to Ammonia. Small 2025, 21, 2412089. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Menzel, J.P.; Li, K.; Ye, Z.; Long, Z.; Navid, I.A.; Yang, K.R.; Xiao, Y.; Batista, V.S.; Mi, Z. Nitrate reduction to ammonia catalyzed by GaN/Si photoelectrodes with metal clusters. Nat. Commun. 2025, 16, 3383. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Hu, J.; Li, Y.; Wang, Z.; Yang, P.; Wang, G.; Ma, Q.; Che, Q.; Dai, Y.; et al. Photocatalytic hydrogen evolution on P-type tetragonal zircon BiVO4. Appl. Catal. B Environ. 2019, 251, 94–101. [Google Scholar] [CrossRef]
- Sun, D.; Bai, H.; Zhao, Y.; Zhang, Q.; Bai, Y.; Liu, Y.; Pang, X.; Wang, F.; Ding, J.; Xu, D.; et al. Amorphous MnCO3/C Double Layers Decorated on BiVO4 Photoelectrodes to Boost Nitrogen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 52763–52770. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Structural and electronic properties of oxygen defective and Se-doped p-type BiVO4(001) thin film for the applications of photocatalysis. Appl. Catal. B Environ. 2018, 224, 895–903. [Google Scholar] [CrossRef]
- Wang, F.; Ding, Q.; Bai, Y.; Bai, H.; Wang, S.; Fan, W. Fabrication of an amorphous metal oxide/p-BiVO4 photocathode: Understanding the role of entropy for reducing nitrate to ammonia. Inorg. Chem. Front. 2022, 9, 805–813. [Google Scholar] [CrossRef]
- Wang, F.; Ding, Q.; Ding, J.; Bai, Y.; Bai, H.; Fan, W. Frustrated Lewis pairs boosting photoelectrochemical nitrate reduction over ZnIn2S4/BiVO4 heterostructure. Chem. Eng. J. 2022, 450, 138260. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Yao, L.; Dan, R.; Sivula, K.; Grätzel, M.; Hagfeldt, A. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Commun. 2020, 11, 318. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J. Nanopixelated Cuprous Oxide Photocathodes for Durable Photoelectrochemical Water Splitting. ACS Energy Lett. 2022, 7, 3244–3250. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Tilley, S.D. Will Cuprous Oxide Really Make It in Water-Splitting Applications? ACS Energy Lett. 2023, 8, 2338–2344. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, F.; Li, J.; Zeng, G.; Ye, Y.; Larson, D.M.; Yano, J.; Crumlin, E.J.; Ager, J.W.; Wang, L.-W.; et al. Investigation and mitigation of degradation mechanisms in Cu2O photoelectrodes for CO2 reduction to ethylene. Nat. Energy 2021, 6, 1124–1132. [Google Scholar] [CrossRef]
- Kim, H.E.; Wi, D.H.; Lee, J.S.; Choi, K.-S. Photoelectrochemical Nitrate and Nitrite Reduction Using Cu2O Photocathodes. ACS Energy Lett. 2024, 9, 1993–1999. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Y.; Cao, S.; Zhang, Y.; Luo, D.; Gao, J.; Li, Z.; Sun, L.; Hou, J. Simultaneous Photoelectrocatalytic Oxidation and Nitrite-Ammonia Conversion with Artificial Photoelectrochemistry Cells. Adv. Energy Mater. 2022, 12, 2201782. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, 1903378. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, M.; Qin, D.; Han, S. Recent advances and perspective of modified TiO2-based photoanodes toward photoelectrochemical water splitting. Fuel 2024, 373, 132366. [Google Scholar] [CrossRef]
- Silveira, V.R.; Fernandes, D.F.; Bericat-Vadell, R.; Edvinsson, T.; Kubart, T.; Sá, J. Phase-dependent photo-assisted electrocatalytic conversion of nitrate to ammonia using TiO2: Insights into amorphous and rutile activity. Appl. Catal. O Open 2024, 197, 207017. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Yu, L.; Nguyen, T.H.; Ikeda, S.; Jiang, F. Over 1% Efficient Unbiased Stable Solar Water Splitting Based on a Sprayed Cu2ZnSnS4 Photocathode Protected by a HfO2 Photocorrosion-Resistant Film. ACS Energy Lett. 2018, 3, 1875–1881. [Google Scholar] [CrossRef]
- Guo, P.; Tang, Y.; Cheng, J.; Mo, R.; Luo, J.; Li, H. Improving Cu2ZnSnS4-Based Photocathodes for Solar Water Splitting via SnO2 Overlayers. ACS Energy Lett. 2024, 9, 6055–6063. [Google Scholar] [CrossRef]
- Jiang, F.; Gunawan; Harada, T.; Kuang, Y.; Minegishi, T.; Domen, K.; Ikeda, S. Pt/In2S3/CdS/Cu2ZnSnS4 Thin Film as an Efficient and Stable Photocathode for Water Reduction under Sunlight Radiation. J. Am. Chem. Soc. 2015, 137, 13691–13697. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, K.; Toe, C.Y.; Yin, J.; Huang, J.; Zeng, Y.; Zhang, D.; Chen, W.; Mohammed, O.F.; Hao, X.; et al. Engineering a Kesterite-Based Photocathode for Photoelectrochemical Ammonia Synthesis from NOx Reduction. Adv. Mater. 2022, 34, 2201670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cho, H.-H.; Yum, J.-H.; Mensi, M.; Sivula, K. An Organic Semiconductor Photoelectrochemical Tandem Cell for Solar Water Splitting. Adv. Energy Mater. 2022, 12, 2202363. [Google Scholar] [CrossRef]
- Oka, K.; Winther-Jensen, B.; Nishide, H. Organic π-Conjugated Polymers as Photocathode Materials for Visible-Light-Enhanced Hydrogen and Hydrogen Peroxide Production from Water. Adv. Energy Mater. 2021, 11, 2003724. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Fan, W.; Ma, W.; Liang, Z.; Shi, J.; Liao, S.; Li, C. Photoassisted Oxygen Reduction Reaction in H2–O2 Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 14748–14751. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Su, X.; Fang, Y.; He, X.; Shan, B. Manipulating Photoconduction in Supramolecular Networks for Solar-Driven Nitrate Conversion to Ammonia and Oxygen. J. Am. Chem. Soc. 2024, 146, 25200–25210. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, M.; Gao, Y.; Wen, Y.; Shan, B. Static Organic p-n Junctions in Photoelectrodes for Solar Ammonia Production with 86 % Internal Quantum Efficiency. Angew. Chem. Int. Ed. 2025, 64, e202415729. [Google Scholar] [CrossRef]
- Sfyri, G.; Kumar, C.V.; Wang, Y.-L.; Xu, Z.-X.; Krontiras, C.A.; Lianos, P. Tetra methyl substituted Cu(II) phthalocyanine as alternative hole transporting material for organometal halide perovskite solar cells. Appl. Surf. Sci. 2016, 360, 767–771. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Chen, Y.; Fu, Y.; Su, J.; Guo, L. Hybrid nanostructured Copper(II) phthalocyanine/TiO2 films with efficient photoelectrochemical performance. Chem. Eng. J. 2020, 382, 122783. [Google Scholar] [CrossRef]
- Moon, H.S.; Yong, K. Noble-metal free photocatalytic hydrogen generation of CuPc/TiO2 nanoparticles under visible-light irradiation. Appl. Surf. Sci. 2020, 530, 147215. [Google Scholar] [CrossRef]
- Li, X.; Fan, W.; Bai, Y.; Liu, Y.; Wang, F.; Bai, H.; Shi, W. Photoelectrochemical reduction of nitrate to ammonia over CuPc/CeO2 heterostructure: Understanding the synergistic effect between oxygen vacancies and Ce sites. Chem. Eng. J. 2022, 433, 133225. [Google Scholar] [CrossRef]
- Nazir, G.; Lee, S.-Y.; Lee, J.-H.; Rehman, A.; Lee, J.-K.; Seok, S.I.; Park, S.-J. Stabilization of Perovskite Solar Cells: Recent Developments and Future Perspectives. Adv. Mater. 2022, 34, 2204380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.H.; Zhang, Y.; Huang, P.; Chen, Q.; Yang, Z.; Jiang, Y. Optical, Electrical, Thermal, Stress, and Energy Yield Simulations Enhance the Performance and Stability of Perovskite Photovoltaics. Adv. Mater. 2025, e14184. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, G.; Xing, Z.; Liu, J.; Li, W.; Zhang, Q.; Liu, H.; Chen, H. Large-Scale Carbon-Based Perovskite Solar Cells: Scalable Fabrication, Performance Optimization, and Industrialization Pathways. Adv. Mater. 2025, e13332. [Google Scholar] [CrossRef] [PubMed]
- Tayyebi, A.; Mehrotra, R.; Mubarok, M.A.; Kim, J.; Zafari, M.; Tayebi, M.; Oh, D.; Lee, S.-H.; Matthews, J.E.; Lee, S.-W.; et al. Bias-free solar NH3 production by perovskite-based photocathode coupled to valorization of glycerol. Nat. Catal. 2024, 7, 510–521. [Google Scholar] [CrossRef]
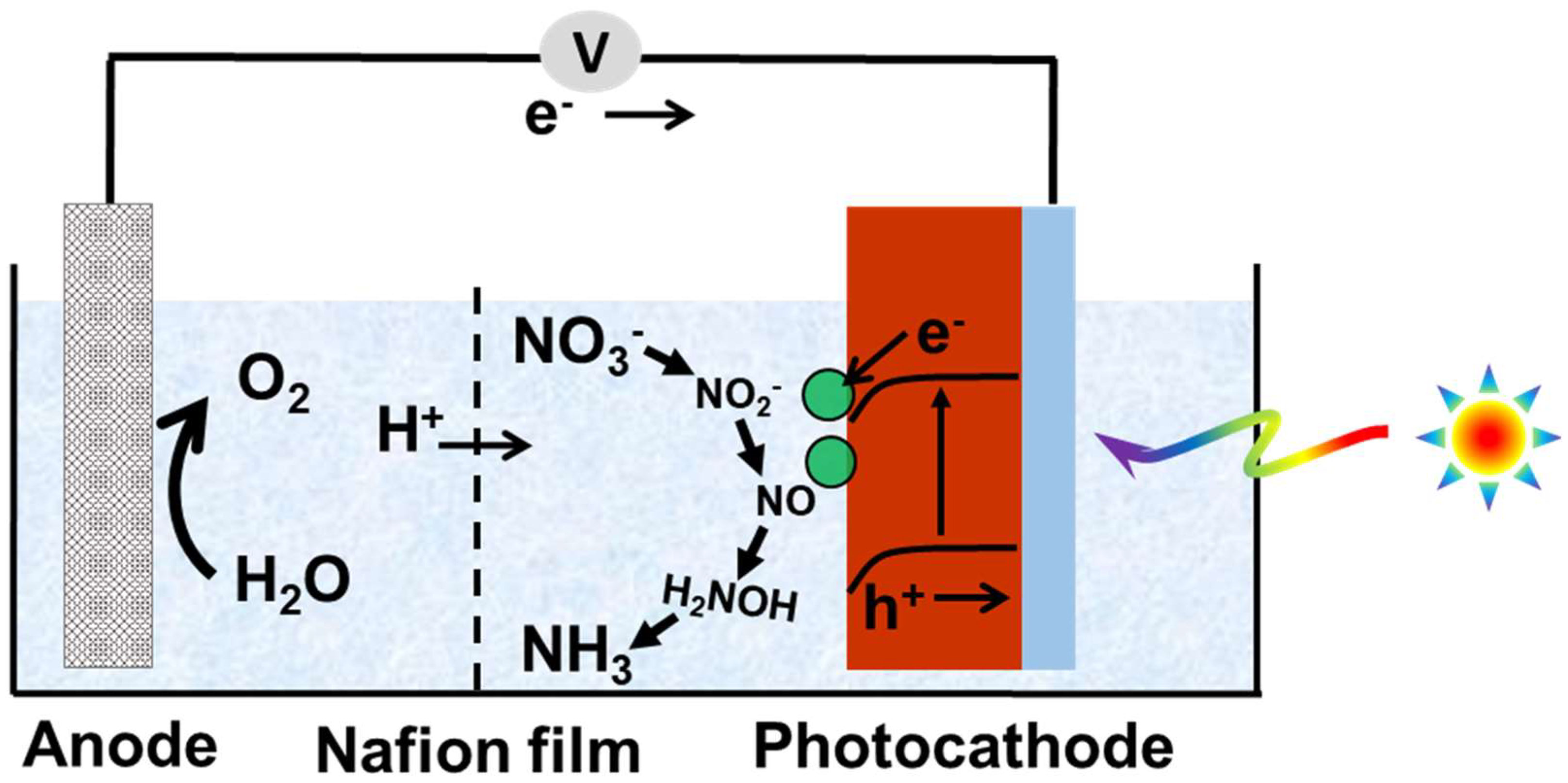
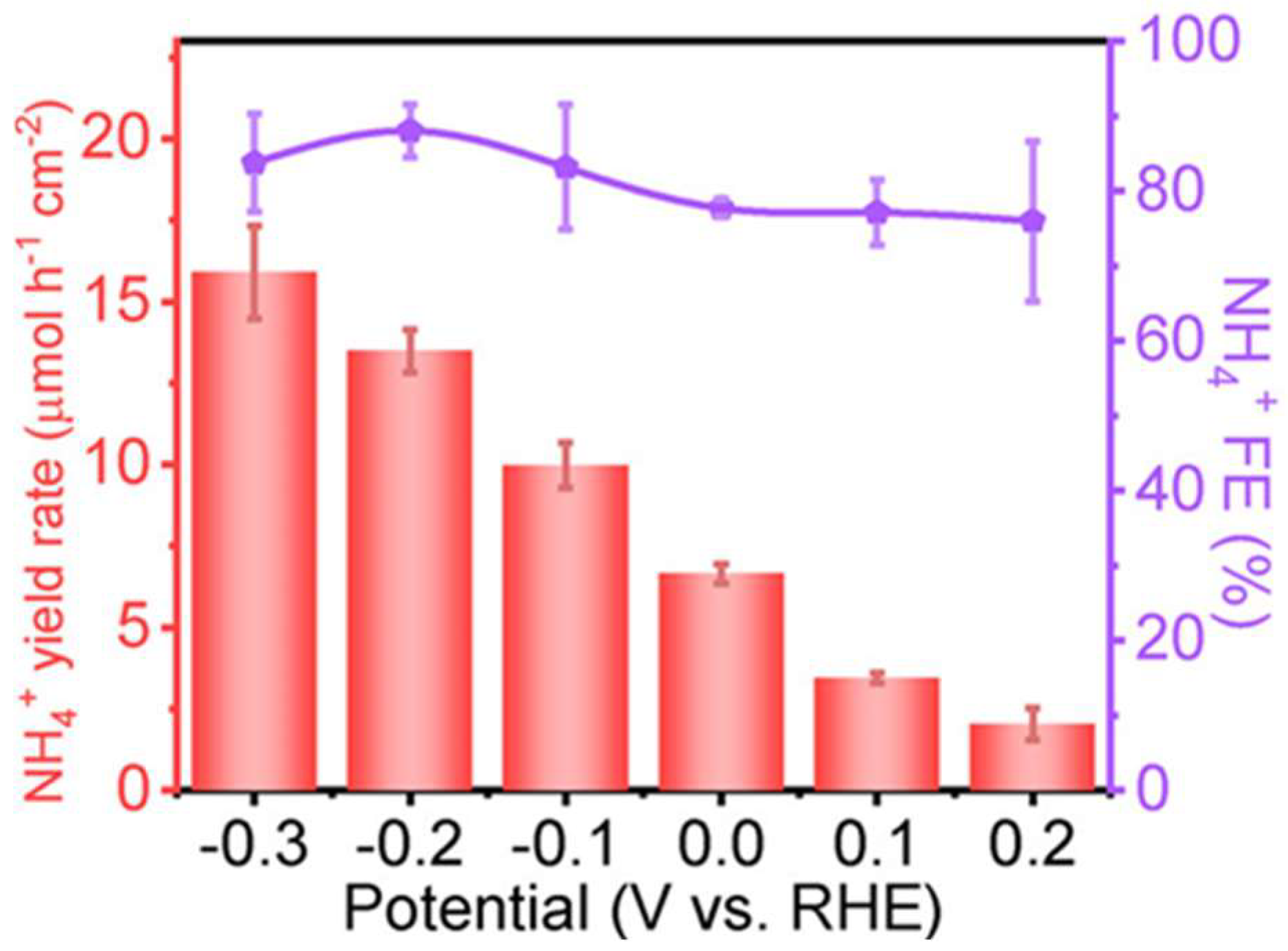
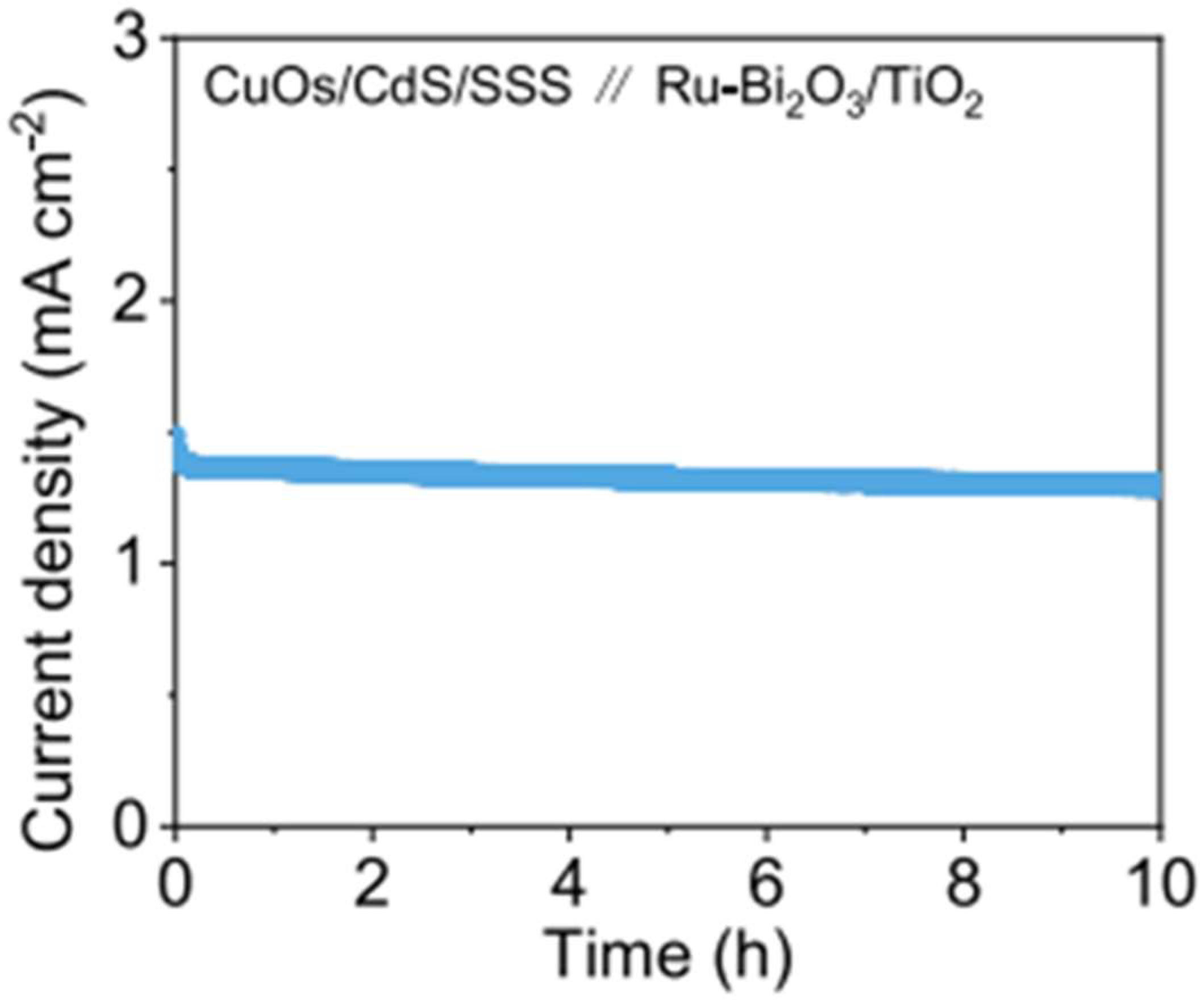
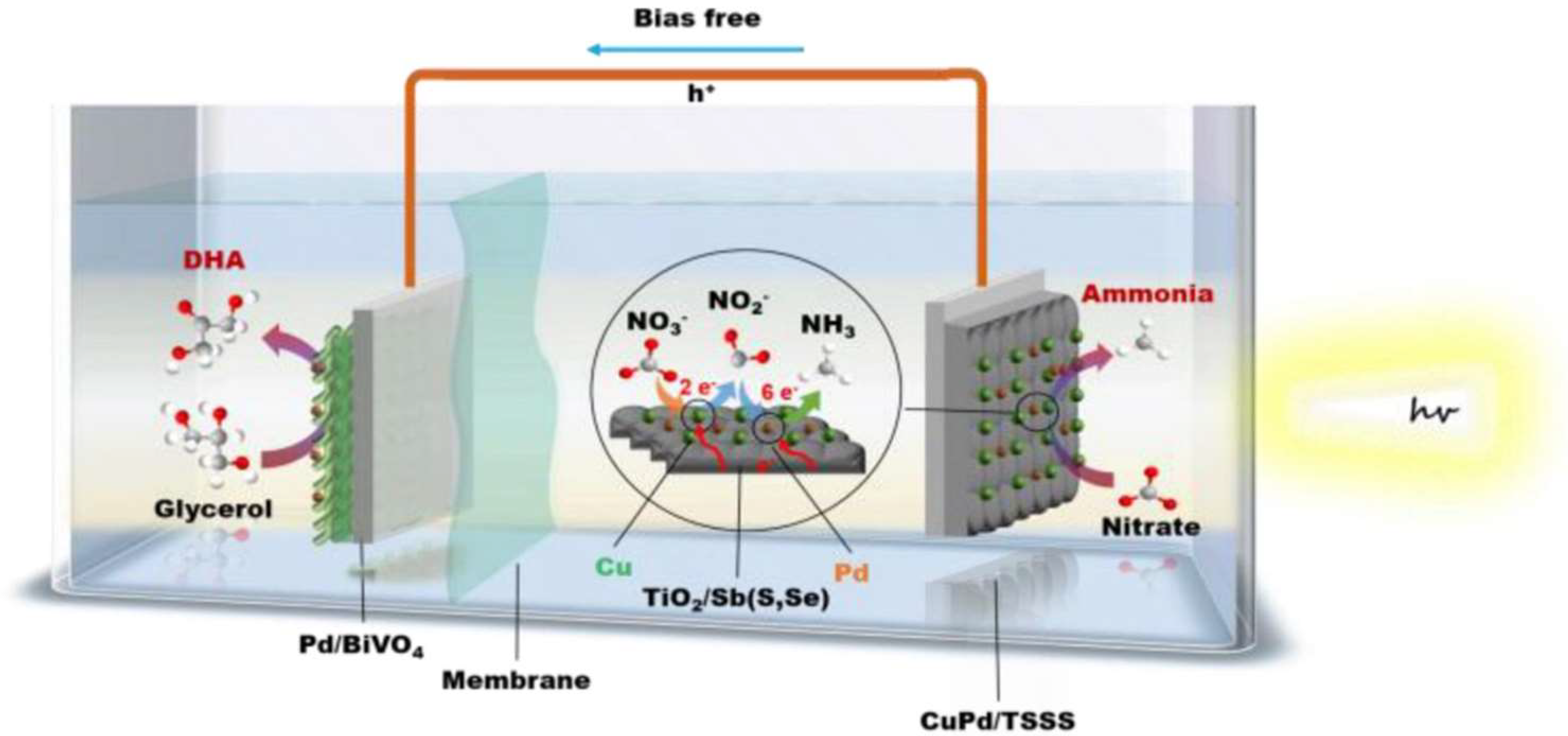
| Reaction Steps | EƟ vs. Standard Hydrogen Electrode (SHE) |
|---|---|
| −0.89 V | |
| — | |
| 1.04 V | |
| −0.47 V | |
| — | |
| −0.78 V | |
| 0.52 V | |
| 0.90 V | |
| 0.42 V |
| Photocathode | Onset Potential (vs. RHE) | Yield Rate of NH3 | Faradaic Efficiency of NH3 | Stability | References |
|---|---|---|---|---|---|
| CoCu/TiO2/Sb2Se3 | 0.43 V | 15.91 μmol h−1 cm−2 at −0.3 V vs. RHE | 88.01% at −0.2 V vs. RHE | 4 h | [47] |
| CuSn/TiO2/Sb2S3 | 0.62 V | 16.96 μmol h−1 cm−2 at 0 V vs. RHE | 97.82% at 0.4 V vs. RHE | 4 h | [54] |
| CuOs/TiO2/CdS/Sb2(S,Se)3 | 0.86 V | 19.87 μmol h−1 cm−2 at 0.1 V vs. RHE | 96.98% at 0.6 V vs. RHE | 12 h | [60] |
| CuPd/TiO2/Sb2(S,Se)3 | 0.83 V | 14.5 μmol h−1 cm−2 at 0 V vs. RHE | 94.6% at 0.8 V vs. RHE | 6 h | [61] |
| Si@TiO2 | — | 63.17 umol h−1 cm−2 at −0.6 V vs. RHE | 94.3% at −0.6 V vs. RHE | 10 h | [67] |
| O_SiNW/Au | 0.3 V | 0.41 umol h−1 cm−2 at 0.1 V vs. RHE | 95.6% at 0.2 V vs. RHE | 8 h | [68] |
| Co0.95Ni0.05/Si | 0.42 V | 120.82 umol h−1 cm−2 at −0.1 V vs. RHE | 98.6% at −0.1 V vs. RHE | 6 h | [69] |
| Cu–Si NW | 0.3 V | 65.91 μmol h−1 cm−2 at −0.6 V vs. RHE | 97.03% at −0.4 V vs. RHE | 36 h | [70] |
| Ni(OH)2@Ni foil/c-Si | 0.69 V | 145.1 umol h−1 cm−2 at −0.1 V vs. RHE | 85% at −0.1 V vs. RHE | 5 h | [71] |
| Si/Cu-NSTL/Co(OH)2 | 1.0 V | 106.6 μmol h−1 cm−2 at 0 V vs. RHE | nearly 100% at 0 V vs. RHE | 10 h | [75] |
| Au/GaN/Si | −0.2 V | 131.1 μmol h−1 cm−2 at −0.8 V vs. RHE | 91.8% at −0.4 V vs. RHE | 8 h | [79] |
| Co/GaN/Si or Ni/GaN/Si | 0.3 V | 201.6 μmol h−1 cm−2 at −0.4 V vs. RHE | 99% at 0.2 V vs. RHE | 10 h | [80] |
| CoFeMnO/BiVO4 | — | 1.04 umol h−1 cm−2 at −0.1 V vs. RHE | 32.8% at −0.1 V vs. RHE | 12 h | [84] |
| ZnIn2S4/BiVO4 | — | 1.76 μmo h−1 cm−2 at −0.1 V vs. RHE | 37.2% at −0.1 V vs. RHE | 13 h | [85] |
| Cu2O | 0.7 V | — | 15% | 5 h | [92] |
| TiO2/AZO/Cu2O/Au | — | — | 98.1% | 1 h | [93] |
| TiOₓ/CdS/CZTS | 0.38 V | 8.21 μmol h−1 cm−2 at −0.2 V vs. RHE | 89.1% at 0.1 V vs. RHE | 5 h | [100] |
| PPV-Cu | — | — | 95% | — | [104] |
| OPN–CuCo | — | — | 96% | — | [105] |
| CuPc/CeO2 | — | 1.16 umol h−1 cm−2 at −0.6 V vs. RHE | 33% at −0.6 V vs. RHE | 2 h | [109] |
| Ru@TiNS/Ni/ Cs0.05(FA0.83MA0.17)0.95Pb(Br0.17I0.83)3 | 1.5 V | — | 93.7% at 0.62 V vs. RHE | 24 h | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhu, K.; Zhang, H. Recent Advances in Photoelectrochemical Nitrate Reduction to Ammonia. Int. J. Mol. Sci. 2026, 27, 470. https://doi.org/10.3390/ijms27010470
Zhu K, Zhang H. Recent Advances in Photoelectrochemical Nitrate Reduction to Ammonia. International Journal of Molecular Sciences. 2026; 27(1):470. https://doi.org/10.3390/ijms27010470
Chicago/Turabian StyleZhu, Kaixin, and Hefeng Zhang. 2026. "Recent Advances in Photoelectrochemical Nitrate Reduction to Ammonia" International Journal of Molecular Sciences 27, no. 1: 470. https://doi.org/10.3390/ijms27010470
APA StyleZhu, K., & Zhang, H. (2026). Recent Advances in Photoelectrochemical Nitrate Reduction to Ammonia. International Journal of Molecular Sciences, 27(1), 470. https://doi.org/10.3390/ijms27010470





