Effect of New Water-Soluble Organosilicon Derivatives of Cartolin-2 on the Germination of Spring Common Wheat Seeds (Triticum aestivum L.)
Abstract
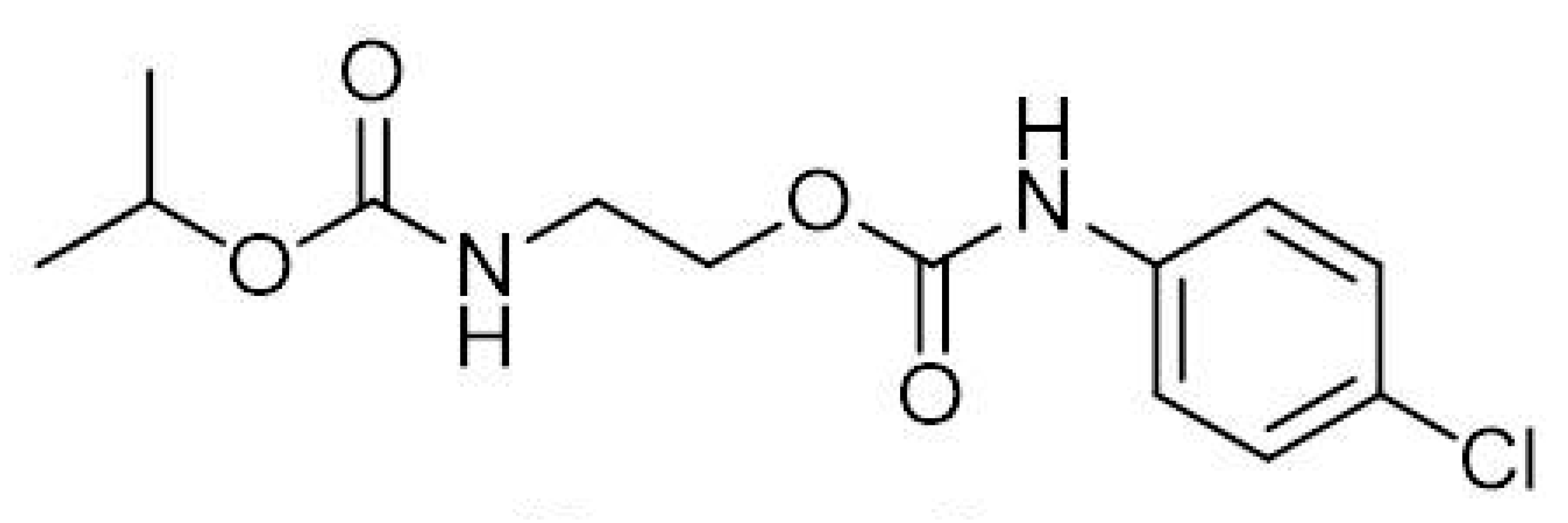
1. Introduction
2. Results and Discussion
2.1. Laboratory Experiments
2.2. Field Test
3. Materials and Methods
3.1. Laboratory Test
3.2. Field Test
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kochetkov, K.A.; Gorunova, O.N.; Bystrova, N.A. Biologically Oriented Hybrids of Indole and Hydantoin Derivatives. Molecules 2023, 28, 602–613. [Google Scholar] [CrossRef]
- Hanaa, H.; Safaa, A. Foliar application of IAA at different growth stages and their influenced on growth and productivity of bread Wheat (Triticum aestivum L.). J. Phys Conf. Ser. 2019, 1294, 092029. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol. Biol. Rep. 2022, 49, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkov, M.S.; Kalistratova, A.V.; Savelieva, E.M.; Romanov, G.A.; Bystrova, N.A.; Kochetkov, K.A. Natural and Synthetic Cytokinins and Their Applications in Biotechnology, Agrochemistry and Medicine. Russ. Chem. Rev. 2020, 89, 787–810. [Google Scholar] [CrossRef]
- Ali, Q.; Shahid, S.; Nazar, N.; Hussain, A.I.; Ali, S.; Chatha, S.A.S.; Hussain, S.M. Use of phytohormones in conferring tolerance to environmental stress. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 245–355. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Zaheer, M.S.; Farrukh, M.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Iqbal, R. Drought ameliorating effect of exogenous applied cytokinin in wheat. Pak. J. Agric. Sci. 2020, 57, 725–733. [Google Scholar]
- Albrecht, T.; Argueso, C.T. Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann. Bot. 2017, 119, 725–735. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Erinle, K.O.; Iqbal, R.; Ahmad, S. Effect of rhizobacteria and cytokinins application on wheat growth and yield under normal vs drought conditions. Commun. Soil Sci. Plant Anal. 2019, 50, 2521–2533. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Rizwan, A.; Riffat, Y.; Madiha, M.; Ambreen, A.; Mazhar, M.; Rehman, A.; Umbreen, S.; Ahmad, M. Phytohormones as Plant Growth Regulators and Safe Protectors against Biotic and Abiotic Stress. Plant Cell Rep. 2022, 40, 1301–1303. [Google Scholar] [CrossRef]
- Baskakov, Y.A.; Shevelukha, V.S.; Simonov, V.D.; Kulaeva, O.N.; Chimishkyan, A.L.; Butenko, R.G.; Shapovalov, A.A.; Nedelchenko, B.M.; Shanbanovich, G.N.; Taschi, V.P.; et al. Carbamoyl Derivatives of Alkanolamines as Plant Growth Regulators. Bulletin of Inventions. Patent SU 1707015 A1, 23 January 1992. [Google Scholar]
- Shapovalov, A.A.; Zubkova, N.F. Domestic growth regulators are growing. Agric. Chem. 2003, 11, 33–47. [Google Scholar] [CrossRef]
- Kovalenko, L.V.; Kalistratova, A.V.; Oshchepkov, M.S.; Solovieva, I.N.; Polivanova, A.G.; Bystrova, N.A.; Kochetkov, K.A. Biological activity of the novel plant growth regulators: N-Alkoxycarbonylaminoethyl-N’-arylureas. Bulg. J. Agric. Sci. 2020, 26, 772–776. [Google Scholar]
- Oshchepkov, M.S.; Kalistratova, A.V.; Kovalenko, L.V.; Ivanova, M.S.; Tsvetikova, M.A.; Bystrova, N.A.; Kochetkov, K.A. Evaluation of potential and rate of the germination of wheat seeds (Triticum aestivum L.) treated with bifunctional growth regulators under water stress. Emir. J. Food Agric. 2023, 35, 1–6. [Google Scholar] [CrossRef]
- Dorairaj, D.; Ismail, M.R. Distribution of silicified microstructures, regulation of cinnamyl alcohol dehydrogenase and lodging resistance in silicon and paclobutrazol mediated Oryza sativa. Front. Physiol. 2017, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Takahashi, E. Chapter 4—Effect of silicate fertilizer application on paddy rice. In Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 49–61. [Google Scholar]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon improves water use efficiency in maize plants. J. Plant Nutr. 2005, 27, 1457–1470. [Google Scholar] [CrossRef]
- Oshchepkov, M.S.; Kovalenko, L.V.; Kalistratova, A.V.; Solovieva, I.N.; Tsvetikova, M.A.; Gorunova, O.N.; Bystrova, N.A.; Kochetkov, K.A. Phytoactive Aryl Carbamates and Ureas as Cytokinin-like Analogs of EDU. Agronomy 2023, 13, 778–790. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, X.; Zhu, Q.; Chao, M.; Fu, Y.; Hu, H. Neodymium Nitrate Improves the Germination of Aged Wheat Seeds by Increasing Soluble Substances and Activating Antioxidative and Metabolic Enzymes in Seeds. Agronomy 2023, 13, 2370. [Google Scholar] [CrossRef]
- Hatsig, S.V.; Frisch, M.; Breuer, F.; Nesi, N.; Ducournau, S.; Wagner, M.-H.; Snowdon, R.J. Genome-wide association mapping unravels the genetic control of seed germination and vigor in Brassica napus. Front. Plant Sci. 2015, 6, 221. [Google Scholar] [CrossRef]
- ISTA. Chapter 1. International Rules for Seed Testing. Certificates 2025, 1, 1–14. Available online: https://www.seedtest.org/api/rm/8888V88928FJ969/ista-rules-2025-01-certificates-web.pdf (accessed on 8 September 2025).
- Halder, T.; Liu, H.; Chen, Y.; Yan, G.; Siddique, K.H.M. Identification of Candidate Genes for Root Traits Using Genotype–Phenotype Association Analysis of Near-Isogenic Lines in Hexaploid Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 3579. [Google Scholar] [CrossRef] [PubMed]
- Aycan, M.; Baslam, M.; Ozdemir, B.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Direct contribution of the maternal genotype on the transgenerational salinity tolerance in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2022, 192, 104648. [Google Scholar] [CrossRef]
- Kayaçetin, F. Assessment of safflower genotypes for individual and combined effects of drought and salinity stress at early seedling growth stages. Turk. J. Agric. For. 2022, 46, 601–612. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z.; Zhenfang Li, Z.; Xue, R.; Cui, W.; Sun, S.; Liu, T.; Zeng, R.; Song, Y. Deficiency in silicon transporter lsi1 compromises inducibility of anti-herbivore defense in rice plants. Front. Plant Sci. 2019, 10, 652. [Google Scholar] [CrossRef]
- Gómez-Merino, F.K.; Trejo-Téllez, L.I.; García-Jiménez, A.; Escobar-Sepúlveda, H.F.; Ramírez-Olvera, S.M. Silicon flow from root to shoot in pepper: A comprehensive in silico analysis reveals a potential linkage between gene expression and hormone signaling that stimulates plant growth and metabolism. PeerJ 2020, 8, e10053. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, 149344. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Wang, R.; Zhao, Y. Recent advances in auxin research in rice and their implications for crop improvement. J. Exp. Bot. 2018, 69, 255–263. [Google Scholar] [CrossRef]
- Vissenberg, K.; Claeijs, N.; Balcerowicz, D.; Schoenaers, S. Hormonal regulation of root hair growth and responses to the environment in Arabidopsis. J. Exp. Bot. 2020, 71, 2412–2427. [Google Scholar] [CrossRef]
- Orzoł, A.; Cruzado-Tafur, E.; Gołebiowski, A.; Rogowska, A.; Pomastowski, P.; Górecki, R.J.; Buszewski, B.; Szultka-Mły’nska, M.; Głowacka, K. Comprehensive Study of Si-Based Compounds in Selected Plants (Pisums ativum L., Medicago sativa L., Triticum aestivum L.). Molecules 2023, 28, 4311. [Google Scholar] [CrossRef]
- Roman, M.; Hlisnikovský, L.; Menšík, L.; Zemanová, V.; Kunzová, E. Temporal trends in winter wheat yield: The role of NPK-fertilization and climate over decades of field experiments. J. Agric. Sci. 2025, 163, 3–12. [Google Scholar] [CrossRef]
- Anderson, B.A.; Sikervar, V. Hexamethyldisilazan. In Encyclopedia of Reagents for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Rosinformagrotech. State Register of Selection Achievements Approved for Use.V.1. “Varieties of Plants” (Official Annual Information); Rosinformagrotech: Moscow, Russia, 2023; Volume 1, p. 632. [Google Scholar]
- Bewley, J.D.; Black, M. Viability and Longevity. In Physiology and Biochemistry of Seeds in Relation to Germination; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar] [CrossRef]
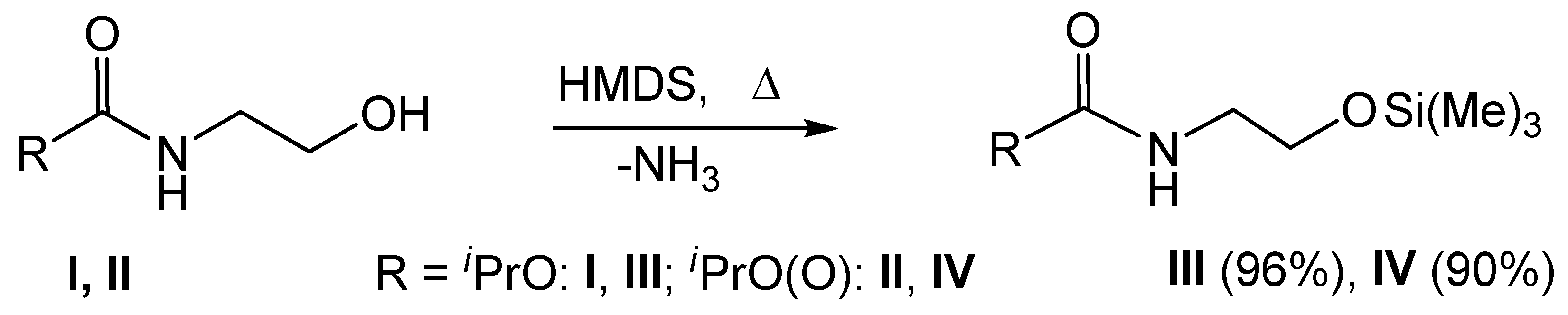
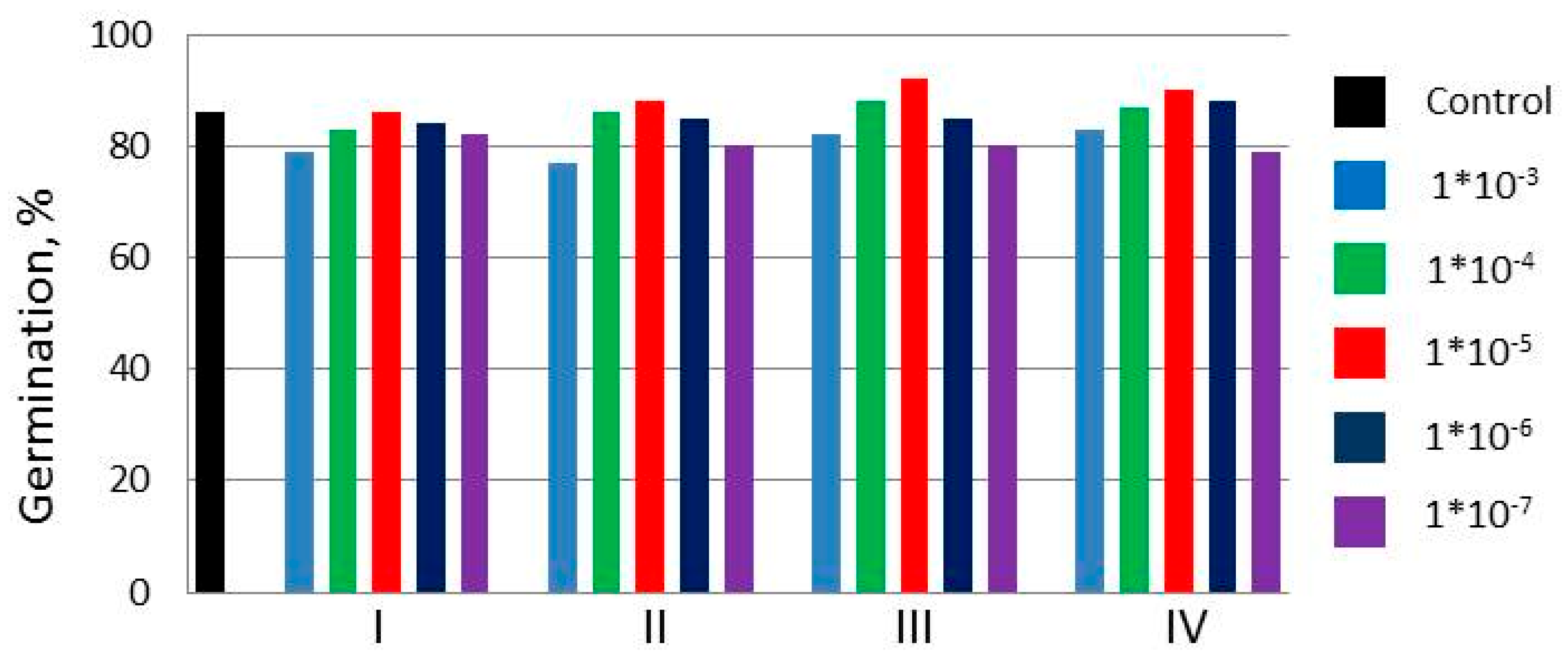

| Gp % | G, % | Root Length, cm | Shoot Height, cm | Green Mass Gain in % Control | |
|---|---|---|---|---|---|
| Control | 40 ± 2.1 | 80 ±1.2 | 7.0 ± 0.8 | 11.5 ± 2.3 | 100 |
| I | 52 ± 2.2 * | 90 ± 2.2 ** | 9.0 ± 0.8 ** | 15.8 ± 2.6 * | 120 ± 2.8 ** |
| II | 56 ± 1.2 ** | 92 ± 2.8 * | 9.7 ± 0.9 * | 16.4 ± 3.2 ** | 123 ± 2.3 * |
| III | 59 ± 1.2 ** | 95 ± 2.2 * | 10.5 ± 1.1 * | 17.2 ± 0.9 * | 125 ± 2.9 * |
| IV | 55 ± 2.2 ** | 93 ± 2.0 * | 9.6 ± 1.5 * | 17.4 ± 3.2 ** | 123 ± 3.6 ** |
| Kin | 58 ± 3.4 * | 94 ± 2.5 ** | 9.2 ± 0.2 ** | 16.3 ± 0.2 * | 124 ±1.6 * |
| IAA | 51 ± 2.5 * | 90 ± 3.8 ** | 9.9 ± 0.3 * | 15.4 ± 0.4 ** | 118 ± 2.7 * |
| Gemination from the Site, % | Plant Height, cm | Quantity of Plant, pcs/m2 | Quantity of Productive Stems, pcs/m2 | Spike Length, cm | Quantity of Grains per Spike | Weight of Grain per Spike, g | Weight of 1000 Grains, g | Yield kg/m2 | Increase g/m2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 68 ±1.73 | 72 ±1.15 | 204 ±3.46 | 816 ±3.64 | 8.0 ±2.02 | 31 ±1.81 | 0.77 ±0.015 | 28.45 ±0.41 | 0.54 ±0.07 | - |
| I | 72 ** ±1.32 | 72 ±0.57 | 216 ±5.19 | 864 * ±5.91 | 9.4 ** ±0.57 | 32 * ±1.54 | 0.74 ±0.026 | 30.13 ±1.10 | 0.85 * ±0.14 | 0.37 ±0.020 |
| II | 73 ** ±1.27 | 78 ±2.3 | 219 ±4.04 | 876 ** ±4.01 | 10.0 ** ±2.30 | 33 ±1.77 | 1.02 ** ±0.018 | 30.13 ±1.20 | 1.05 ±0.22 | 0.57 ±0.031 |
| III | 76 * ±1.72 | 73 ±1.73 | 228 ±2.30 | 912 * ±5.16 | 9.8 * ±0.77 | 32.5 ±1.34 | 0.78 * ±0.028 | 31.80 * ±1.16 | 0.59 ±0.15 | 0.11 ±0.021 |
| IV | 74 ±1.15 | 75 ±2.30 | 231 ±5.19 | 924 * ±4.05 | 10.5 ** ±1.73 | 35.5 * ±1.93 | 0.80 * ±0.031 | 30.96 ±1.34 | 0.73 ±0.12 | 0.25 ±0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kochetkov, K.A.; Gorunova, O.N.; Bystrova, N.A.; Oshchepkov, M.S. Effect of New Water-Soluble Organosilicon Derivatives of Cartolin-2 on the Germination of Spring Common Wheat Seeds (Triticum aestivum L.). Int. J. Mol. Sci. 2026, 27, 469. https://doi.org/10.3390/ijms27010469
Kochetkov KA, Gorunova ON, Bystrova NA, Oshchepkov MS. Effect of New Water-Soluble Organosilicon Derivatives of Cartolin-2 on the Germination of Spring Common Wheat Seeds (Triticum aestivum L.). International Journal of Molecular Sciences. 2026; 27(1):469. https://doi.org/10.3390/ijms27010469
Chicago/Turabian StyleKochetkov, Konstantin A., Olga N. Gorunova, Nataliya A. Bystrova, and Maxim S. Oshchepkov. 2026. "Effect of New Water-Soluble Organosilicon Derivatives of Cartolin-2 on the Germination of Spring Common Wheat Seeds (Triticum aestivum L.)" International Journal of Molecular Sciences 27, no. 1: 469. https://doi.org/10.3390/ijms27010469
APA StyleKochetkov, K. A., Gorunova, O. N., Bystrova, N. A., & Oshchepkov, M. S. (2026). Effect of New Water-Soluble Organosilicon Derivatives of Cartolin-2 on the Germination of Spring Common Wheat Seeds (Triticum aestivum L.). International Journal of Molecular Sciences, 27(1), 469. https://doi.org/10.3390/ijms27010469








